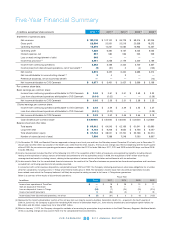
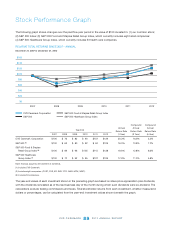
CVS 2012 Annual Report Download - page 83
Download and view the complete annual report
Please find page 83 of the 2012 CVS annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.CVS CAREMARK 2012 ANNUAL REPORT
81
pharmacies to Texas Medicaid for reimbursement. The Company has been providing documents and other information in
response to this request for information.
A purported shareholder derivative action was filed on behalf of nominal defendant CVS Caremark Corporation against
certain of the Company’s officers and members of its Board of Directors. The action was originally filed in June 2012 and,
after the court granted leave to amend the original fling, an amended complaint was filed in November 2012. The amended
complaint alleges a single claim for breach of fiduciary duty relating to the Company’s alleged failure to properly implement
internal regulatory controls to comply with the Controlled Substances Act and the Combat Methamphetamine Epidemic Act.
In November 2012, the Company received a subpoena from the OIG requesting information concerning automatic refill
programs used by pharmacies to refill prescriptions for customers. The Company is cooperating and will be providing
documents and other information in response to this request for information.
Effective January 15, 2013, CMS imposed intermediate sanctions on the Company’s SilverScript Medicare Part D
PDP, consisting of immediate suspension of further plan enrollment and marketing activities. The sanctions relate to
the Company’s compliance with certain Medicare Part D requirements and do not affect the enrollment status of the
Company’s current PDP enrollees. CMS has granted a limited waiver of these sanctions to allow the Company’s PDP to
continue to enroll eligible retirees of existing employer clients into its SilverScript plans and into employer group waiver
plans to fulfill the Company’s commitments to implement and provide employer group waiver plan services. This limited
waiver currently extends through April 30, 2013, and CMS has advised the Company that it will consider further extensions
of the waiver on a rolling basis. At the beginning of the 2013 Medicare Part D plan year, the Company implemented an
enrollment systems conversion process and other actions to consolidate its PDP plans. These consolidation efforts have
impacted the enrollment and coverage determination services the Company provides to PDP enrollees. The Company
is cooperating with CMS to address the service issues resulting from the Company’s plan consolidation efforts and to
develop and implement a corrective action plan to resolve and remove the sanctions. The Company cannot predict how
long the sanctions will remain in effect or the scope of corrective action or other remedial actions that CMS may require
in order for the sanctions to be removed.
The Company is also a party to other legal proceedings and inquiries arising in the normal course of its business, none
of which is expected to be material to the Company. The Company can give no assurance, however, that its business,
financial condition and results of operations will not be materially adversely affected, or that the Company will not be
required to materially change its business practices, based on: (i) future enactment of new health care or other laws or
regulations; (ii) the interpretation or application of existing laws or regulations as they may relate to our business, the
pharmacy services, retail pharmacy or retail clinic industries or to the health care industry generally; (iii) pending or future
federal or state governmental investigations of our business or the pharmacy services, retail pharmacy or retail clinic
industry or of the health care industry generally; (iv) institution of government enforcement actions against us; (v) adverse
developments in any pending
qui tam
lawsuit against us, whether sealed or unsealed, or in any future
qui tam
lawsuit that
may be filed against us; or (vi) adverse developments in other pending or future legal proceedings against us or affecting
the pharmacy services, retail pharmacy or retail clinic industry or the health care industry generally.