Pentax 2006 Annual Report Download - page 25
Download and view the complete annual report
Please find page 25 of the 2006 Pentax annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.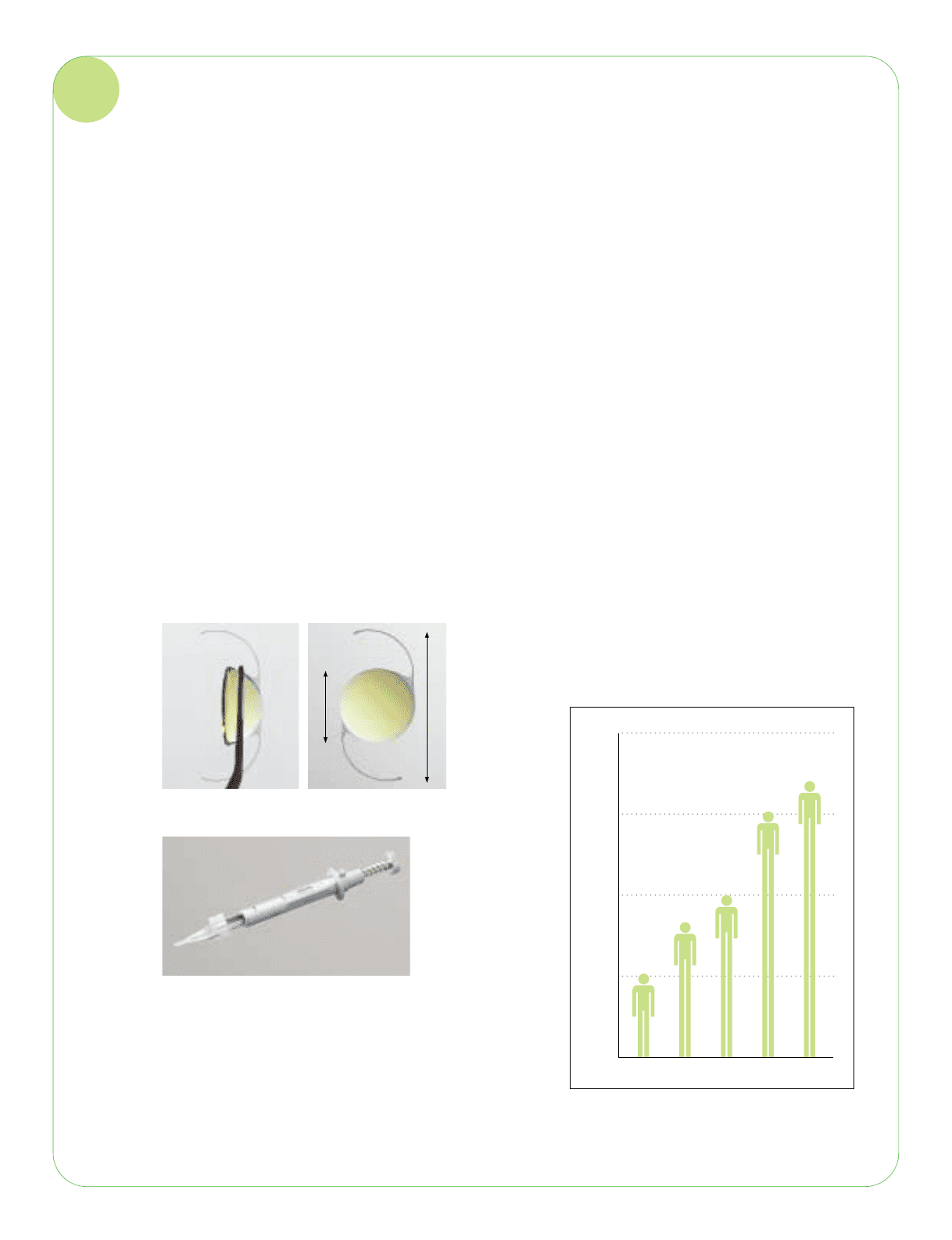
Intraocular Lenses (IOLs)
In Hoya’s Eye Care field, sales of IOLs are expected to record the strongest growth.
IOLs are medical devices used for the surgical treatment of cataracts. After the eye’s clouded lens has been removed, an
IOL, which is an artificial lens, is inserted to restore good vision. Together with the gathering pace of the aging of society,
primarily in developed countries, the number of patients with age-related cataracts is increasing rapidly, and further growth is
expected over the long term. There are two types of IOLs: the conventional hard type and the new foldable soft type, which
is folded for insertion during the cataract operation. This latter type, which can be surgically inserted through a much smaller
incision, is on the way to becoming the mainstream solution due to the greatly reduced discomfort for the patient.
The shift to use of the foldable lens type IOL is proceeding quickly in the Japanese market, and in this market Hoya’s
product quality is valued highly. Market share is growing steadily. In 2004, a new yellow type of IOL was introduced which, in
addition to offering the ability to reduce UV light transmission, also offered a protective effect for the retina. An injector
system that simplifies the surgical procedure has also become firmly established in the market.
During the fiscal year ended March 31, 2006, Hoya worked hard to grow its business in world markets, and was
successful in building stronger networks with ophthalmology clinics and stepping up marketing activities. Driven primarily by
Germany and France, sales in the Company’s European markets grew very significantly. In Asia, Hoya posted solid results in
countries such as China and South Korea. In the United States, the Company is making final preparations to gain Food and
Drug Administration (FDA) certification. It will lodge applications in 2006, with certification expected some time during 2007.
Hoya’s Singapore plant, which commenced commercial operations in 2004, started shipping products to Japan during the
fiscal year ended March 31, 2006, in addition to continuing its exporting to Europe. The plant now serves as a central point of
production for the Company’s global markets.
Eye Care Health Care Division
Source:
Journal of Community Eye Health
International Resource Centre, International Centre of Eye Health
Global Estimate of the Number of Eyes with
< 6/60 Due to Cataracts
(Millions)
200
150
100
50
0
198019902000 20102020
Foldable acrylic IOL: HOYA AF-1 (UY)
IOL injector: HOYA Injector System HOYA-IS
6.0 mm
12.5 mm