CVS 2006 Annual Report Download - page 42
Download and view the complete annual report
Please find page 42 of the 2006 CVS annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.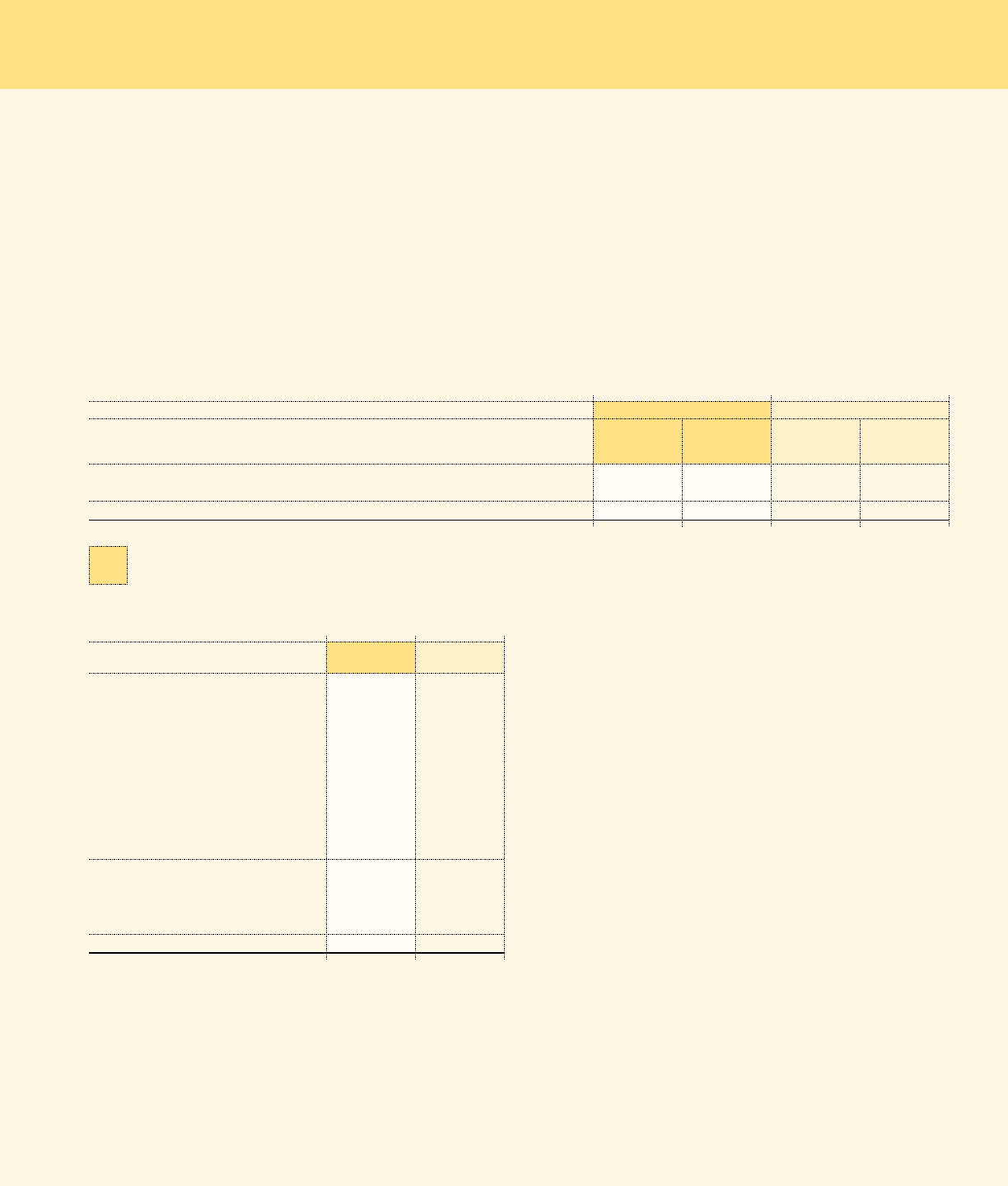
2006 Annual Report 39
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The increase in the gross carrying amount of the Company’s amortizable
intangible assets during 2006 was primarily due to the acquisition of
the Standalone Drug Business. The amortization expense for intangible
assets totaled $161.2 million in 2006, $128.6 million in 2005 and
$95.9 million in 2004. The anticipated annual amortization expense
for these intangible assets is $186.2 million in 2007, $176.7 million
in 2008, $167.0 million in 2009, $157.0 million in 2010 and
$148.6 million in 2011.
The carrying amount of goodwill was $3,195.2 million and
$1,789.9 million as of December 30, 2006 and December 31, 2005,
respectively. During 2006, gross goodwill increased primarily due to
the acquisition of the Standalone Drug Business. There was no
impairment of goodwill during 2006.
Intangible assets other than goodwill are required to be separated into
two categories: finite-lived and indefinite-lived. Intangible assets with
finite useful lives are amortized over their estimated useful lives, while
intangible assets with indefinite useful lives are not amortized. The
Company currently has no intangible assets with indefinite lives.
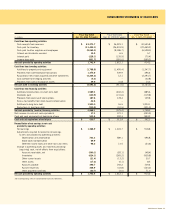
Following is a summary of the Company’s amortizable intangible assets as of the respective balance sheet dates:
Dec. 30, 2006 Dec. 31, 2005
Gross Gross
Carrying Accumulated Carrying Accumulated
In millions Amount Amortization Amount Amortization
Customer lists and Covenants not to compete $ 1,457.6 $ (563.4) $ 1,152.4 $ (435.9)
Favorable leases and Other 552.2 (128.2) 185.5 (99.8)
$ 2,009.8 $ (691.6) $ 1,337.9 $ (535.7)
4 BORROWING AND CREDIT AGREEMENTS
Following is a summary of the Company’s borrowings as of the respective
balance sheet dates:
Dec. 30, Dec. 31,
In millions 2006 2005
Commercial paper $ 1,842.7 $ 253.4
5.625% senior notes due 2006 – 300.0
3.875% senior notes due 2007 300.0 300.0
4.0% senior notes due 2009 650.0 650.0
5.75% senior notes due 2011 800.0 –
4.875% senior notes due 2014 550.0 550.0
6.125% senior notes due 2016 700.0 –
8.52% ESOP notes due 2008 (1) 82.1 114.0
Mortgage notes payable 11.7 21.0
Capital lease obligations 120.9 0.7
5,057.4 2,189.1
Less:
Short-term debt (1,842.7) (253.4)
Current portion of long-term debt (344.3) (341.6)
$ 2,870.4 $ 1,594.1
(1) See Note 6 for further information about the Company’s ESOP Plan.
In connection with our commercial paper program, we maintain a
$675 million, five-year unsecured back-up credit facility, which expires
on June 11, 2009 and a $675 million five-year unsecured back-up
credit facility, which expires on June 2, 2010. In preparation for the
consummation of the acquisition of the Standalone Drug Business, we
entered into a $1.4 billion, five-year unsecured back-up credit facility,
which expires on May 12, 2011. The credit facilities allow for borrowings
at various rates depending on the Company’s public debt ratings and
require the Company to pay a quarterly facility fee of 0.1%, regardless
of usage. As of December 30, 2006, the Company had no outstanding
borrowings against the credit facilities. The weighted average interest
rate for short-term debt was 5.3% and 3.3% as of December 30, 2006
and December 31, 2005, respectively.
On August 15, 2006, the Company issued $800 million of 5.75%
unsecured senior notes due August 15, 2011 and $700 million of
6.125% unsecured senior notes due August 15, 2016 (collectively the
“Notes”). The Notes pay interest semi-annually and may be redeemed
at any time, in whole or in part at a defined redemption price plus
accrued interest. Net proceeds from the Notes were used to repay a
portion of the outstanding commercial paper issued to finance the
acquisition of the Standalone Drug Business.
To manage a portion of the risk associated with potential changes in
market interest rates, during the second quarter of 2006 the Company
entered into forward starting pay fixed rate swaps (the “Swaps”), with
a notional amount of $750 million. The Swaps settled in conjunction
with the placement of the long-term financing, at a loss of $5.3 million.
The Company accounts for the above derivatives in accordance with
SFAS No. 133, “Accounting for Derivative Instruments and Hedging
Activities,” as modified by SFAS No. 138, “Accounting for Derivative
Instruments and Certain Hedging Activities,” which requires the resulting
loss to be recorded in shareholders’ equity as a component of accumulated
other comprehensive loss. This unrealized loss will be amortized as a
component of interest expense over the life of the related long-term
financing. As of December 30, 2006, the Company had no freestanding
derivatives in place.