Quest Diagnostics 2007 Annual Report Download - page 103
Download and view the complete annual report
Please find page 103 of the 2007 Quest Diagnostics annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.The Company is involved in various legal proceedings. Some of the proceedings against the Company
involve claims that are substantial in amount.
NID Investigation
NID and the Company each received a subpoena from the United States Attorney’s Office for the Eastern
District of New York during the fourth quarter of 2004. The subpoenas requested a wide range of business
records, including documents regarding parathyroid hormone (“PTH”) test kits manufactured by NID and PTH
testing performed by the Company. The Company has voluntarily and actively cooperated with the investigation,
providing information, witnesses and business records of NID and the Company, including documents related to
PTH tests and test kits, as well as other tests and test kits. In the second and third quarters of 2005, the FDA
conducted an inspection of NID and issued a Form 483 listing the observations made by the FDA during the
course of the inspection. NID responded to the Form 483.
During the fourth quarter of 2005, NID instituted its second voluntary product hold within a six-month
period, due to quality issues, which adversely impacted the operating performance of NID. As a result, the
Company evaluated a number of strategic options for NID, and on April 19, 2006, decided to cease operations at
NID. Upon completion of the wind-down of operations in the third quarter of 2006, the operations of NID were
classified as discontinued operations. During the third quarter of 2006, the government issued two additional
subpoenas, one to NID and one to the Company. The subpoenas covered various records, including records
related to tests and test kits in addition to PTH.
During the third quarter of 2007, the government and the Company began settlement discussions. In the
course of those discussions, the government disclosed to the Company certain of the government’s legal theories
regarding the amount of damages allegedly incurred by the government, which include alleged violations of civil
and criminal statutes including the False Claims Act and the Food, Drug and Cosmetics Act. Violations of these
statutes and related regulations could lead to a warning letter, injunction, fines or penalties, exclusion from
federal healthcare programs and/or criminal prosecution, as well as claims by third parties. The Company
analyzed the government’s position and presented its own analysis which argued against many of the
government’s claims. In light of that analysis and based on the status of settlement discussions, the Company has
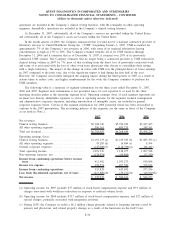
established a reserve, in accordance with generally accepted accounting principles, reflected in discontinued
operations, of $241 million in connection with these claims. Of the total reserve, $51 million and $190 million
were recorded in the third and fourth quarters, respectively, of 2007. The Company estimates that the amount
reserved represents the minimum expected probable loss with respect to this matter. The Company does not
believe that a reasonable estimate for these losses in excess of the established reserve can be made at this time.
The Company has recorded a deferred tax benefit associated with that portion of the reserve that it expects will
be tax deductible. Eventual losses related to these matters may substantially exceed the reserve, and the impact
could be material to the Company’s results of operations, cash flows and financial condition in the period that
such matters are determined or paid.
The Company continues to engage in discussions with the United States Attorney’s Office and those
discussions potentially could lead to an agreement in principle to resolve some or all of the matters in the near
future. There can be no assurance, however, when or whether a settlement may be reached, or as to its terms. If
the Company cannot reach an acceptable settlement agreement with the United States Attorney’s Office, the
Company would defend itself and NID and could incur significant costs in doing so.
Other Matters
The Company has in the past entered into several settlement agreements with various government and
private payers relating to industry-wide billing and marketing practices that had been substantially discontinued.
The federal or state governments may bring additional claims based on new theories as to the Company’s
practices which management believes to be in compliance with law. In addition, certain federal and state statutes,
including the qui tam provisions of the federal False Claims Act, allow private individuals to bring lawsuits
against healthcare companies on behalf of government or private payers alleging inappropriate billing practices.
The Company is aware of certain pending lawsuits, including a class action lawsuit, and has received several
subpoenas related to billing practices.
F-33
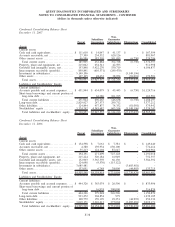
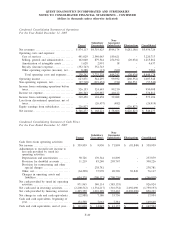
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(dollars in thousands unless otherwise indicated)