Pfizer 2010 Annual Report Download - page 31
Download and view the complete annual report
Please find page 31 of the 2010 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
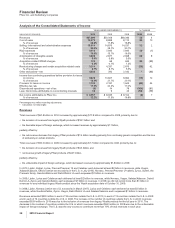
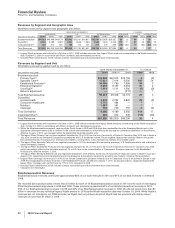
Financial Review
Pfizer Inc. and Subsidiary Companies
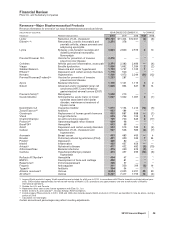
Below are significant regulatory actions by, and filings pending with, the FDA and regulatory authorities in the EU and Japan as well
as new drug candidates and additional indications in late-stage development:
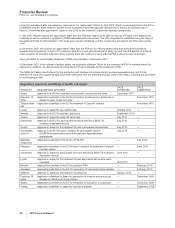
Recent FDA approvals:
PRODUCT INDICATION DATE APPROVED
Prevnar 13 Infant Prevention of invasive pneumococcal disease in infants and young children February 2010
Pending U.S. new drug applications (NDA) and supplemental filings:
PRODUCT INDICATION DATE SUBMITTED
tafamidis meglumine Treatment of transthyretin amyloid polyneuropathy (ATTR-PN) February 2011
Prevnar 13 Adult Prevention of pneumococcal disease in adults 50 years of age and older December 2010
Taliglucerase alfa Treatment of Gaucher disease December 2009
Sutent Pancreatic neuroendocrine tumor December 2009
Genotropin Adult growth hormone deficiency (Mark VII multidose disposable device) October 2009
Celebrex Chronic pain August 2009
Geodon Treatment of bipolar disorder––pediatric filing October 2008
Spiriva Respimat device for chronic obstructive pulmonary disease November 2007
Zmax Treatment of bacterial infections––sustained release––acute otitis media (AOM)
and sinusitis––pediatric filing
November 2006
Viviant Osteoporosis treatment and prevention June 2006
Pristiq Vasomotor symptoms of menopause June 2006
Vfend Treatment of fungal infections––pediatric filing June 2005
On October 6, 2010, we completed the acquisition of FoldRx. Its lead product candidate, tafamidis meglumine (Tafamidis), is in
registration in both the U.S. and the EU as a first-in-class oral therapy for the treatment of transthyretin amyloid polyneuropathy
(ATTR-PN), a progressively fatal genetic neurodegenerative disease, for which liver transplant is the only treatment option currently
available. Tafamidis has orphan drug designation in both the U.S. and EU and fast-track designation in the U.S.
In November 2009, we entered into a license and supply agreement with Protalix BioTherapeutics (Protalix), which provides us
exclusive worldwide rights, except in Israel, to develop and commercialize taliglucerase alfa for the treatment of Gaucher disease. In
April 2010, Protalix completed a rolling NDA with the FDA for taliglucerase alfa. Taliglucerase alfa was granted orphan drug
designation in the U.S. in September 2009. In February 2011, Protalix received a “complete response” letter from the FDA for the
taliglucerase alfa NDA that set forth additional requirements for approval. Protalix will work with the FDA to determine next steps.
In May 2010, the FDA issued a “complete response” letter requesting additional information in connection with our supplemental
NDA seeking approval to use Sutent for the treatment of pancreatic neuroendocrine tumors. We have provided the requested
information, including an analysis of independently reviewed scans, and are working with the FDA to pursue regulatory approval.
In April 2010, we received a “complete response” letter from the FDA for the Genotropin Mark VII multidose disposable device
submission. In August 2010, we submitted our response to address the requests and recommendations included in the FDA letter.
In June 2010, we received a “complete response” letter from the FDA for the Celebrex chronic pain supplemental NDA. We are
working with the FDA to determine the next steps.
In October 2009, we received a “complete response” letter from the FDA with respect to the supplemental NDA for Geodon for the
treatment of acute bipolar mania in children and adolescents aged 10 to 17 years. In October 2010, we submitted our response to
address the issues raised in the FDA letter. In April 2010, we received a “warning letter” from the FDA with respect to the clinical trial
in support of this supplemental NDA. We are working with the FDA to address the issues raised in the letter.
Boehringer Ingelheim (BI), our alliance partner, holds the NDAs for Spiriva Handihaler and Spiriva Respimat. In September 2008, BI
received a “complete response” letter from the FDA for the Spiriva Respimat submission. The FDA is seeking additional data, and
we are coordinating with BI, which is working with the FDA to provide the additional information. A full response will be submitted to
the FDA upon the completion of planned and ongoing studies.
In September 2007, we received an “approvable” letter from the FDA for Zmax that set forth requirements to obtain approval for the
pediatric acute otitis media (AOM) indication based on pharmacokinetic data. A supplemental filing for pediatric AOM and sinusitis
remains under review.
Two “approvable” letters were received by Wyeth in April and December 2007 from the FDA for Viviant (bazedoxifene), for the
prevention of post-menopausal osteoporosis, that set forth the additional requirements for approval. In May 2008, Wyeth received an
“approvable” letter from the FDA for the treatment of post-menopausal osteoporosis. The FDA is seeking additional data, and we
have been systematically working through these requirements and seeking to address the FDA’s concerns. In February 2008, the
FDA advised Wyeth that it expects to convene an advisory committee to review the pending NDAs for both the treatment and
2010 Financial Report 29