Pfizer 2010 Annual Report Download - page 20
Download and view the complete annual report
Please find page 20 of the 2010 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
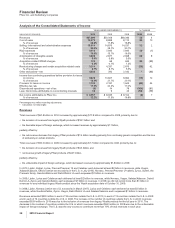
The amounts recorded for the major components of acquired identifiable intangible assets are as follows:
(MILLIONS OF DOLLARS)
AMOUNTS
RECOGNIZED
AS OF
ACQUISITION
DATE
WEIGHTED-
AVERAGE
USEFUL
LIVES
(YEARS)
Developed technology rights—finite-lived $27,065 12
Brands—finite-lived 615 14
Brands—indefinite-lived 7,993 —
In-process research and development—indefinite-lived(a) 13,822 —
Other—finite-lived 389 4
Total $49,884
(a) Includes $9.9 billion associated with Prevnar/Prevenar 13 Infant. Prevenar 13 Infant was approved by the EU member states in December 2009 and
as a result, was reclassified to Developed technology rights––finite-lived. Prevnar 13 Infant was approved in the U.S. in February 2010.
ODeveloped Technology Rights—Developed technology rights include the right to develop, use, market, sell and/or offer for sale a
product, compound or other intellectual property that we have acquired with respect to products, compounds and/or processes that
have been completed. Developed Technology Rights acquired include Enbrel, and to a lesser extent, Premarin and Effexor, among
others. As of the acquisition date, Prevnar/Prevenar 13 Infant was classified in IPR&D, but received regulatory approval in a major
market in December 2009. As a result, we reclassified the asset from IPR&D to Developed Technology Rights—finite-lived and
began to amortize the asset.
OBrands—Brands generally represent the value associated with tradenames and know-how, as the products themselves usually no
longer receive patent protection. Brands acquired include Advil, Centrum, Robitussin, Caltrate, ChapStick, Preparation H, 1st Age
Nutrition, 2nd Age Nutrition and 3rd Age Nutrition, among others.
OIn-Process Research and Development—IPR&D intangible assets represent the right to develop, use, sell and/or offer for sale a
compound or other intellectual property that we have acquired with respect to compounds and/or processes that have not been
completed or approved. These assets are required to be classified as indefinite-lived assets until the successful completion or
abandonment of the associated research and development efforts. Accordingly, during the development period after the date of
acquisition, these assets will not be amortized until approval is obtained in a major market, typically either the U.S. or the EU, or in a
series of other countries, subject to certain specified conditions and management judgment. At that time, we will determine the useful
life of the asset, reclassify the asset out of IPR&D and begin amortization. The useful life of an amortizing asset generally is
determined by identifying the period in which substantially all of the cash flows are expected to be generated.
If the associated research and development effort is abandoned, the related IPR&D assets likely will be written off, and we will record
an impairment loss in our consolidated statements of income.
As of the acquisition date, IPR&D included Prevnar/Prevenar 13 Infant (see below), and to a lesser extent, Prevnar/Prevenar 13
Adult, and Neratinib (treatment of cancer), among others (see the “Analysis of Consolidated Statements of Income: Product
Developments––Biopharmaceutical: New Drug Candidates in Late-Stage Development” section of this Financial Review). In
December 2009, Prevnar/Prevenar 13 Infant received regulatory approval in a major market and, as a result, we reclassified the
asset from IPR&D to Developed Technology Rights and began to amortize the asset.
The fair value of finite-lived identifiable intangible assets will be recognized in our results of operations over the expected useful life
of the individual assets.
Some of the more significant estimates and assumptions inherent in the estimate of the fair value of identifiable intangible assets
include all assumptions associated with forecasting product profitability from the perspective of a market participant.
Specifically:
ORevenue—We use historical, forecast, industry or other sources of market data, including estimates of the number of units to be
sold, selling prices, market penetration, market share and year-over-year growth rates over the product’s life cycle.
OCost of sales, Sales and marketing expenses, General and administrative expenses—We use historical, forecast, industry or other
sources of market data.
OR&D expenses—In the case of approved products, we estimate the appropriate level of ongoing R&D support, and for unapproved
compounds, we estimate the amount and timing of costs to develop the R&D into viable products.
OEstimated life of the asset—We assess the asset’s life cycle and the competitive trends impacting the asset, including consideration
of any technical, legal, regulatory or economic barriers to entry, as well as expected changes in standards of practice for indications
addressed by the asset.
OInherent risk—We use a discount rate that is based on the weighted-average cost of capital with an additional premium to reflect the
risks associated with the specific intangible asset, such as country risks (political, inflation, currency and property risks) and
commercial risks. In addition, for unapproved assets, an additional risk factor is added for the risk of technical and regulatory
success, called the probability of technical and regulatory success (PTRS).
18 2010 Financial Report