Pfizer 2008 Annual Report Download - page 72
Download and view the complete annual report
Please find page 72 of the 2008 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
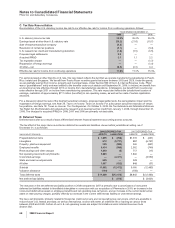
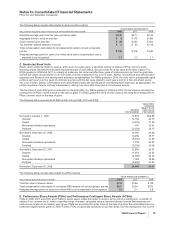
B. Other Intangible Assets
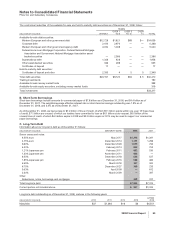
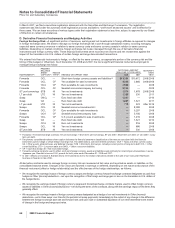
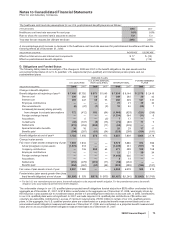
The components of identifiable intangible assets, primarily included in our Pharmaceutical segment, as of December 31 follow:
2008 2007
(MILLIONS OF DOLLARS)
GROSS
CARRYING
AMOUNT
ACCUMULATED
AMORTIZATION
IDENTIFIABLE
INTANGIBLE
ASSETS, LESS
ACCUMULATED
AMORTIZATION
GROSS
CARRYING
AMOUNT
ACCUMULATED
AMORTIZATION
IDENTIFIABLE
INTANGIBLE
ASSETS, LESS
ACCUMULATED
AMORTIZATION
Finite-lived intangible
assets:
Developed technology
rights $31,484 $(17,673) $13,811 $32,433 $(15,830) $16,603
Brands 1,016 (487) 529 1,017 (452) 565
License agreements 246 (78) 168 212 (59) 153
Trademarks 118 (78) 40 128 (82) 46
Other(a) 531 (291) 240 459 (264) 195
Total amortized finite-lived
intangible assets 33,395 (18,607) 14,788 34,249 (16,687) 17,562
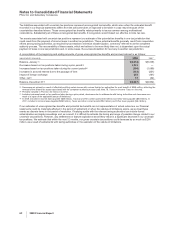
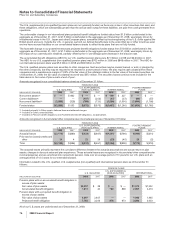
Indefinite-lived intangible
assets:
Brands 2,860 — 2,860 2,864 — 2,864
Trademarks 70 — 70 71 — 71
Other 3—31—1
Total indefinite-lived
intangible assets 2,933 — 2,933 2,936 — 2,936
Total identifiable intangible
assets $36,328 $(18,607) $17,721(b) $37,185 $(16,687) $20,498
(a) Includes patents, non-compete agreements, customer contracts and other intangible assets.
(b) Decrease was primarily related to amortization and the impact of foreign exchange, partially offset by acquisitions.
Developed technology rights represent the amortized value associated with developed technology, which has been acquired from
third parties and which can include the right to develop, use, market, sell and/or offer for sale the product, compounds and
intellectual property that we have acquired with respect to products, compounds and/or processes that have been completed. We
possess a well-diversified portfolio of hundreds of developed technology rights across therapeutic categories, primarily representing
the commercialized products included in our Pharmaceutical segment that we acquired in connection with our Pharmacia acquisition
in 2003. While the Arthritis and Pain therapeutic category represents about 29% of the total amortized value of developed
technology rights as of December 31, 2008, the balance of the amortized value is distributed in a range of 5% to 15% across the
following Pharmaceutical therapeutic product categories: Ophthalmology; Oncology; Urology; Infectious and Respiratory Diseases;
Endocrine Disorders categories; and as a group, the Cardiovascular and Metabolic Diseases, Central Nervous System Disorders
and All Other categories. The significant components include values determined for Celebrex, Detrol/Detrol LA, Xalatan, Genotropin
and Zyvox. Also included in this category are the post-approval milestone payments made under our alliance agreements for certain
Pharmaceutical products, such as Rebif and Spiriva. These rights are all subject to our review for impairment, explained in Note 1K.
Significant Accounting Policies: Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets.
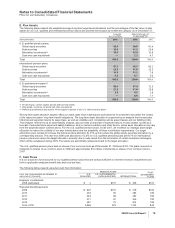
The weighted-average life of our total finite-lived intangible assets is approximately seven years, which includes developed
technology rights at eight years. Total amortization expense for finite-lived intangible assets was $2.8 billion in 2008, $3.2 billion in
2007 and $3.4 billion in 2006.
Brands represent the amortized value associated with tradenames, as the products themselves no longer receive patent protection.
Most of these assets are associated with our Pharmaceutical segment and the significant components include values determined for
Depo-Provera, Xanax and Medrol.
In 2007, we recorded charges of $1.1 billion in Cost of sales and Selling, informational and administrative expenses related to the
impairment of Exubera (included in our Pharmaceutical segment) (see Note 4D. Certain Charges: Exubera). In 2006, we recorded
charges of $320 million in Other (income)/deductions—net related to the impairment of our Depo-Provera brand, a contraceptive
injection (included in our Pharmaceutical segment).
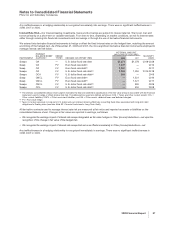
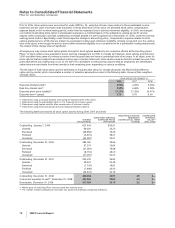
The annual amortization expense expected for the years 2009 through 2013 is as follows:
(MILLIONS OF DOLLARS) 2009 2010 2011 2012 2013
Amortization expense $2,459 $2,446 $2,421 $2,077 $1,727
70 2008 Financial Report