Pfizer 2008 Annual Report Download - page 24
Download and view the complete annual report
Please find page 24 of the 2008 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc and Subsidiary Companies
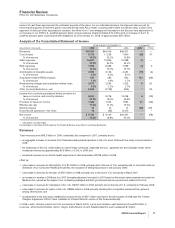
The decrease in Lipitor worldwide revenues in 2008 compared to 2007 was driven by a combination of factors, including the
following:
Othe impact of an intensely competitive lipid-lowering market, with competition from multi-source generic simvastatin and branded
products in the U.S.;
Oincreased payer pressure in the U.S.; and
Oslower growth in the lipid-lowering market, due in part to a slower rate of growth in the Medicare Part D population and heightened
overall patient cost-sensitivity in the U.S., resulting in a softening overall market demand,
partially offset by:
Othe favorable impact of foreign exchange; and
Ooperational growth internationally.
See Notes to Consolidated Financial Statements—Note 19. Legal Proceedings and Contingencies for a discussion of recent
developments with respect to certain patent and product litigation relating to Lipitor.
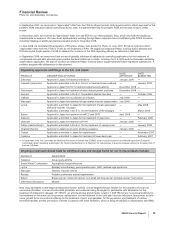
•Norvasc, for treating hypertension, lost exclusivity in the U.S. in March 2007. Norvasc also experienced patent expirations in most
other major markets, with the exception of Canada. Norvasc worldwide revenues in 2008 decreased 25% compared to 2007.
See Notes to Consolidated Financial Statements—Note 19. Legal Proceedings and Contingencies for a discussion of recent
developments with respect to certain patent litigation relating to Norvasc.
•Chantix/Champix, the first new prescription treatment to aid smoking cessation in nearly a decade, became available to patients in the
U.S. in August 2006 and in select E.U. markets in December 2006 and has been launched in all major markets. Chantix/Champix has
been prescribed to more than ten million patients globally since its launch. Chantix/Champix recorded worldwide revenues of $846
million in 2008, a decrease of 4% compared to 2007. In the U.S., revenues of $489 million in 2008 declined 30% compared to the same
period in 2007 following changes to the Chantix U.S. label during 2008. Internationally, revenues of $357 million in 2008 increased 95%
compared to 2007, due primarily to launches in additional countries and continued growth in the U.K., Spain, Canada, Belgium and
Japan.
In January 2008, we added a warning to Chantix’s label in the U.S. that patients who are attempting to quit smoking by taking
Chantix should be observed by a physician for neuropsychiatric symptoms like changes in behavior, agitation, depressed mood,
suicidal ideation and suicidal behavior. A causal relationship between Chantix and these reported symptoms has not been
established.
In May 2008, we updated the Chantix label in the U.S. to provide further guidance about the use of Chantix. The updated label
advises that patients should stop taking Chantix and contact their healthcare provider immediately if agitation, depressed mood,
or changes in behavior that are not typical for them are observed, or if they develop suicidal thoughts or suicidal behavior.
U.S. prescription trends and U.S. revenues for Chantix have declined following the addition of the warnings to the product’s label
in the U.S. We are continuing our educational and promotional efforts, which are focused on the Chantix benefit-risk proposition,
the significant health consequences of smoking and the importance of the physician-patient dialogue in helping patients quit
smoking. In September 2008, the U.S. branded direct-to-consumer campaign was relaunched with print, television and web
advertising.
See Notes to Consolidated Financial Statements—Note 19. Legal Proceedings and Contingencies for a discussion of recent
developments with respect to certain product litigation relating to Chantix.
•Caduet, a single pill therapy combining Norvasc and Lipitor, recorded worldwide revenues of $589 million, an increase of 4% for 2008,
compared to 2007, due primarily to growth in new launch countries, partially offset by lower revenues in the U.S., due to the introduction
of generic amlodipine besylate and increased competition in the hypertension market. A more focused message platform and highly
targeted consumer campaign have recently stabilized the rate of new patient starts in the U.S.
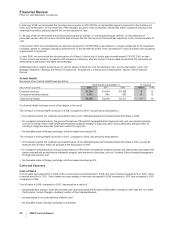
•Lyrica, indicated for the management of post-herpetic neuralgia (PHN), diabetic peripheral neuropathy (DPN) and fibromyalgia, and as
adjunctive therapy for adult patients with partial onset seizures in the U.S., and for neuropathic pain and general anxiety disorder (GAD)
outside the U.S., recorded worldwide revenues of $2.6 billion in 2008, an increase of 41% compared to 2007. In June 2007, Lyrica was
approved in the U.S. for the management of fibromyalgia, one of the most common chronic, widespread pain conditions, which affects
more than five million Americans. Lyrica is the leading branded treatment for fibromyalgia, PHN and DPN in the U.S.
In July 2008, an FDA advisory committee concurred with the FDA's finding of a potential increased signal regarding suicidal
thoughts and behavior for the class of 11 epilepsy drugs reviewed, including Lyrica and Neurontin. However, the committee
determined that the available data did not warrant black box labeling as had been recommended by the FDA. We are confident in
the efficacy and safety profile of Lyrica and Neurontin for their approved indications. We have conducted an extensive review of
controlled clinical trials and post-marketing reports for both medicines, which showed no evidence of an increased signal
22 2008 Financial Report