Pfizer 2008 Annual Report Download - page 55
Download and view the complete annual report
Please find page 55 of the 2008 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
we use an estimated allocation factor (based on historical payments) and total revenues by country against our actual invoiced sales to
project the expected level of reimbursement. We obtain third-party information that helps us to monitor the adequacy of these accruals.
•Provisions for pharmaceutical chargebacks (primarily reimbursements to wholesalers for honoring contracted prices to third parties)
closely approximate actual as we settle these deductions generally within two to four weeks of incurring the liability.
•Provisions for pharmaceutical returns are based on a calculation at each market that incorporates the following, as appropriate: local
returns policies and practices; returns as a percentage of sales; an understanding of the reasons for past returns; estimated shelf-life by
product; an estimate of the amount of time between shipment and return or lag time; and any other factors that could impact the
estimate of future returns, such as loss of exclusivity, product recalls, or a changing competitive environment, as appropriate.
•We record sales incentives as a reduction of revenues at the time the related revenues are recorded or when the incentive is offered,
whichever is later. We estimate the cost of our sales incentives based on our historical experience with similar incentives programs.
•Our accruals for Medicaid rebates, Medicare rebates, performance-based contract rebates and chargebacks were $1.5 billion as of
December 31, 2008, and $1.4 billion as of December 31, 2007, and are included in Other current liabilities.
Taxes collected from customers relating to product sales and remitted to governmental authorities are presented on a net basis; that
is, they are excluded from Revenues.
Alliances—We have agreements to co-promote pharmaceutical products discovered by other companies. Alliance revenues are
earned when our co-promotion partners ship the related product and title passes to their customer. Alliance revenues are primarily
based upon a percentage of our co-promotion partners’ net sales. Expenses for selling and marketing these products are included in
Selling, informational and administrative expenses.
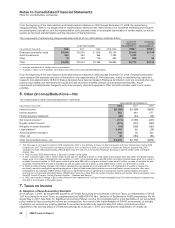
H. Cost of Sales and Inventories
We value inventories at lower of cost or market. Cost is determined as follows:
•finished goods and work in process at average actual cost; and
•raw materials and supplies at average or latest actual cost.
I. Selling, Informational and Administrative Expenses
Selling, informational and administrative costs are expensed as incurred. Among other things, these expenses include the costs of
marketing, advertising, shipping and handling, information technology and non-manufacturing employee compensation.
Advertising expenses relating to production costs are expensed as incurred and the costs of radio time, television time and space in
publications are expensed when the related advertising occurs. Advertising expenses totaled approximately $2.6 billion in 2008,
$2.7 billion in 2007 and $2.6 billion in 2006.
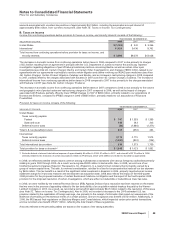
J. Research and Development Expenses
Research and development (R&D) costs are expensed as incurred. These expenses include the costs of our proprietary R&D
efforts, as well as costs incurred in connection with our third-party collaboration efforts. Before a compound receives regulatory
approval, we record milestone payments made by us to third parties under contracted R&D arrangements as expense when the
specific milestone has been achieved. Once a compound receives regulatory approval, we record any subsequent milestone
payments in Identifiable intangible assets, less accumulated amortization and, unless the assets are determined to have an
indefinite life, we amortize them evenly over the remaining agreement term or the expected product life cycle, whichever is shorter.
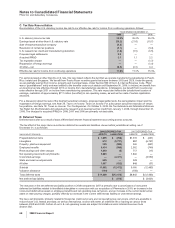
K. Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets
Long-lived assets include:
•Goodwill—Goodwill represents the excess of the purchase price of an acquired business over the assigned values of its net assets.
Goodwill is not amortized.
•Identifiable intangible assets, less accumulated amortization—These acquired assets are recorded at our cost. Intangible assets with
finite lives are amortized evenly over their estimated useful lives. Intangible assets with indefinite lives are not amortized.
•Property, plant and equipment, less accumulated depreciation—These assets are recorded at original cost and increased by the cost of
any significant improvements after purchase. We depreciate the cost evenly over the assets’ estimated useful lives. For tax purposes,
accelerated depreciation methods are used as allowed by tax laws.
Amortization expense related to acquired intangible assets that contribute to our ability to sell, manufacture, research, market and
distribute products, compounds and intellectual property are included in Amortization of intangible assets as they benefit multiple
business functions. Amortization expense related to intangible assets that are associated with a single function and depreciation of
property, plant and equipment are included in Cost of sales, Selling, informational and administrative expenses and Research and
development expenses, as appropriate.
We review all of our long-lived assets, including goodwill and other intangible assets, for impairment indicators at least annually and
we perform detailed impairment testing for goodwill and indefinite-lived assets annually and for all other long-lived assets whenever
impairment indicators are present. When necessary, we record charges for impairments of long-lived assets for the amount by which
the present value of future cash flows, or some other fair value measure, is less than the carrying value of these assets. The process
2008 Financial Report 53