Pfizer 2008 Annual Report Download - page 29
Download and view the complete annual report
Please find page 29 of the 2008 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
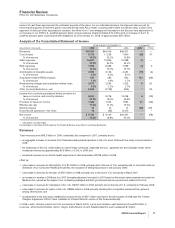
Financial Review
Pfizer Inc and Subsidiary Companies
partially offset by:
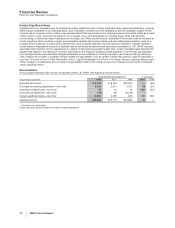
•the impact of higher implementation costs associated with our cost-reduction initiatives of $745 million in 2008, compared to $700
million in 2007.
Cost of sales in 2007, compared to 2006, increased as a result of:
•asset impairment charges, write-offs and other exit costs associated with Exubera of $2.6 billion recorded in 2007 (see the “Our 2008
Performance: Certain Charges—Exubera” section of this Financial Review);
•the unfavorable impact of foreign exchange on expenses;
•the impact of higher implementation costs associated with our cost-reduction initiatives of $700 million in 2007, compared to $392
million in 2006; and
•costs of $194 million for 2007, related to business transition activities associated with the sale of our Consumer Healthcare business,
completed in December 2006,
partially offset by:
•savings related to our cost-reduction initiatives.
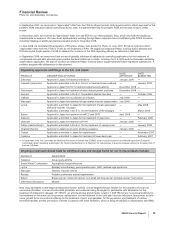
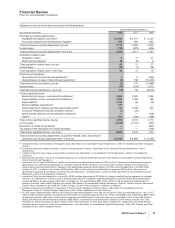
Selling, Informational and Administrative (SI&A) Expenses
SI&A expenses decreased 7% in 2008, compared to 2007, which reflects:
•savings related to our cost-reduction initiatives; and
•charges associated with Exubera of $85 million recorded in 2007 (see the “Our 2008 Performance: Certain Charges—Exubera” section
of this Financial Review),
partially offset by:
•the unfavorable impact of foreign exchange on expenses; and
•the impact of higher implementation costs associated with our cost-reduction initiatives of $413 million in 2008, compared to $334
million in 2007.
SI&A expenses in 2007 were comparable to 2006, which reflects:
•savings related to our cost-reduction initiatives,
offset by:
•the unfavorable impact of foreign exchange on expenses;
•the impact of higher implementation costs associated with our cost-reduction initiatives of $334 million in 2007, compared to $243
million in 2006; and
•charges associated with Exubera of $85 million recorded in 2007 (see the “Our 2008 Performance: Certain Charges—Exubera” section
of this Financial Review).
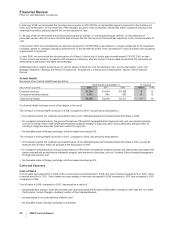
Research and Development (R&D) Expenses
R&D expenses decreased 2% in 2008, compared to 2007, which reflects:
•the up-front payment to Bristol-Myers Squibb Company (BMS) of $250 million and additional payments to BMS related to product
development efforts, in connection with our collaboration to develop and commercialize apixaban, recorded in 2007;
•exit costs, such as contract termination costs, associated with Exubera of $100 million recorded in 2007 (see the “Our 2008
Performance: Certain Charges—Exubera” section of this Financial Review); and
•savings related to our cost-reduction initiatives,
2008 Financial Report 27