Pfizer 2006 Annual Report Download - page 80
Download and view the complete annual report
Please find page 80 of the 2006 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
2006 Financial Report 79
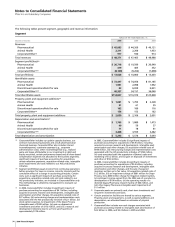
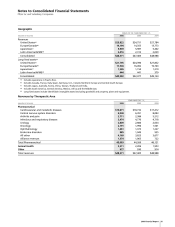
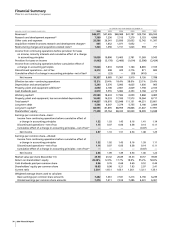
Financial Summary
Pfizer Inc and Subsidiary Companies
On April 16, 2003, Pfizer acquired Pharmacia Corporation in a
transaction accounted for as a purchase. All financial information
reflects the following as discontinued operations: our Consumer
Healthcare, in-vitro allergy and autoimmune diagnostic testing, certain
European generics, surgical ophthalmic, confectionery, shaving and fish-
care products businesses and the femhrt, Loestrin and Estrostep
women’s health product lines, as applicable.
In addition, depreciation and amortization includes amortization of
goodwill prior to our adoption of SFAS No. 142, Goodwill and Other
Intangible Assets, in 2002.
(a)
In 2001, we brought the accounting methodology pertaining to
accruals for estimated liabilities related to Medicaid discounts and
contract rebates of Warner-Lambert into conformity with our
historical method. This adjustment increased revenues in 2001 by
$175 million. 2001 data reflects reclassifications between
Revenues and Other costs and expenses of $108 million, as a result
of the January 1, 2002, adoption of EITF Issue No. 00-25, Vendor
Income Statement Characterization of Consideration Paid to a
Reseller of the Vendor’s Products.
(b)
Research and development expenses includes co-promotion
charges and milestone payments for intellectual property rights of
$292 million in 2006: $156 million in 2005; $160 million in 2004;
$380 million in 2003; $32 million in 2002; and $206 million in
2001.
(c)
In 2006, 2005, 2004 and 2003, we recorded charges for the
estimated portion of the purchase price of acquisitions allocated
to in-process research and development.
(d)
Restructuring charges and acquisition-related costs primarily
includes the following:
2006 — Restructuring charges of $1.3 billion related to our AtS
productivity initiative.
2005 — Integration costs of $532 million and restructuring
charges of $372 million related to our acquisition of Pharmacia in
2003 and restructuring charges of $438 million related to our AtS
productivity initiative.
2004 — Integration costs of $454 million and restructuring
charges of $680 million related to our acquisition of Pharmacia in
2003.
2003 — Integration costs of $808 million and restructuring
charges of $166 million related to our acquisition of Pharmacia in
2003.
2002 — Integration costs of $333 million and restructuring
charges of $167 million related to our merger with Warner-
Lambert in 2000 and pre-integration costs of $94 million related
to our pending acquisition of Pharmacia.
2001 — Integration costs of $428 million and restructuring
charges of $329 million related to our merger with Warner-
Lambert in 2000.
(e)
In 2005, as a result of adopting FIN 47, Accounting for Conditional
Asset Retirement Obligations, we recorded a non-cash pre-tax
charge of $40 million ($23 million, net of tax). In 2003, as a result
of adopting SFAS No. 143, Accounting for Asset Retirement
Obligations, we recorded a non-cash pre-tax charge of $47 million
($30 million, net of tax).
In 2002, as a result of adopting SFAS No. 142, Goodwill and Other
Intangible Assets, we recorded pre-tax charges of $565 million
($410 million, net of tax).
(f)
Includes discontinued operations, (see Notes to Consolidated
Financial Statements—Note 20. Segment, Geographic and
Revenue Information.)
(g)
For 2005 through 2001, includes assets held for sale of our
Consumer Healthcare business, and for 2004 through 2001, also
includes in-vitro allergy and autoimmune diagnostic testing,
surgical ophthalmic, certain European generics, confectionery and
shaving businesses (and the Tetra business in 2001) and the
femhrt, Loestrin and Estrostep women’s health product lines.
(h)
Defined as long-term debt, deferred taxes, minority interests and
shareholders’ equity.