Pfizer 2006 Annual Report Download - page 71
Download and view the complete annual report
Please find page 71 of the 2006 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
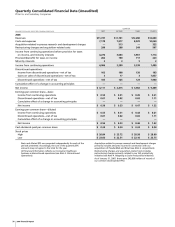
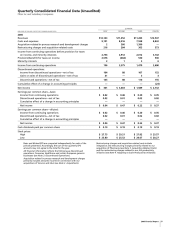
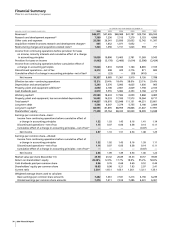
70 2006 Financial Report
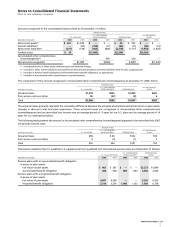
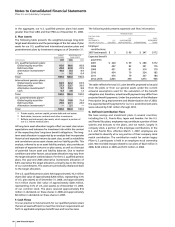
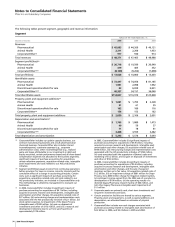
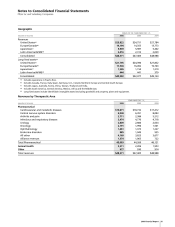
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
This litigation originally included both individual actions as well as
various purported nationwide and statewide class actions. However,
as the result of the voluntary dismissal of certain purported class
actions and the withdrawal of the class action allegations by the
plaintiffs in certain other actions, this litigation now consists of
individual actions and a few purported statewide class actions.
Viagra
A number of lawsuits, including purported class actions, have been
filed against us in various federal and state courts alleging that
Viagra causes certain types of visual injuries. The plaintiffs in the
purported class actions seek to represent nationwide and certain
statewide classes of Viagra users. All of the actions seek damages
for personal injury, and the purported class actions also seek
medical monitoring. In January 2006, the federal court cases
were transferred for consolidated pre-trial proceedings to a
Multi-District Litigation (In re Viagra Products Liability Litigation
MDL-1724) in the U.S. District Court for the District of Minnesota.
Zoloft
A number of individual lawsuits have been filed against us in
various federal and state courts alleging personal injury, including
suicide and suicide attempt in certain cases, as a result of the
purported ingesting of Zoloft.
C. Consumer and Commercial Matters
Neurontin
A number of lawsuits, including purported class actions, have been
filed against us in various federal and state courts alleging claims
arising from the promotion and sale of Neurontin. The plaintiffs
in the purported class actions seek to represent nationwide and
certain statewide classes consisting of persons, including
individuals, health insurers, employee benefit plans and other
third-party payors, who purchased or reimbursed patients for
the purchase of Neurontin that allegedly was used for indications
other than those included in the product labeling approved by the
FDA. In October 2004, many of the suits pending in federal courts,
including individual actions as well as purported class actions, were
transferred for consolidated pre-trial proceedings to a Multi-
District Litigation (In re Neurontin Marketing, Sales Practices and
Product Liability Litigation MDL-1629) in the U.S. District Court for
the District of Massachusetts. Purported class actions also have
been filed against us in various Canadian provincial courts alleging
claims arising from the promotion and sale of Neurontin.
A number of individual lawsuits have been filed against us in
various U.S. federal and state courts and in certain other countries
alleging personal injury, including suicide and suicide attempt in
certain cases, as a result of the purported ingesting of Neurontin.
Certain of the federal court actions have been transferred for
consolidated pre-trial proceedings to the same Multi-District
Litigation referred to in the preceding paragraph.
Lipitor
Beginning in September 2005, three purported class actions were
filed against us in various federal courts alleging claims relating to
the promotion of Lipitor. In January 2006, two of the actions were
voluntarily dismissed without prejudice. In the remaining action,
which is pending in the U.S. District Court for the Southern District
of Florida, the plaintiffs seek to represent a nationwide class
consisting of women (regardless of age) and men over age 65
who in each case had no history of heart disease or diabetes and
who purchased Lipitor within four years before the filing of the
action. The plaintiffs allege that the Company engaged in false and
misleading advertising in violation of state consumer protection
laws by allegedly promoting Lipitor for the prevention of heart
disease in the aforementioned two groups. The action seeks
monetary and injunctive relief, including treble damages. In
addition, a purported class action on behalf of residents of the
Province of Quebec has been filed against us in Canada that asserts
claims under Canadian law and seeks relief substantially similar to
the claims asserted and the relief sought in the U.S. action.
Separately, in March and April 2006, six purported class actions
were filed against us in various federal courts alleging claims
relating to the promotion of Lipitor. In May 2006, five of the
actions were voluntarily dismissed without prejudice, and the
plaintiffs in those actions were added as plaintiffs in the remaining
action. The complaint in the remaining action, which is pending
in the U.S. District Court for the Northern District of Illinois,
alleges that, through patient and medical education programs and
other actions, the Company promoted Lipitor for use by certain
patients contrary to cholesterol guidelines, which are referenced
in the product labeling, that recommend changes to diet and
exercise. The plaintiffs seek to represent nationwide and certain
statewide classes consisting of health and welfare funds and
other third-party payors that purchased Lipitor for such patients
or reimbursed such patients for the purchase of Lipitor since
January 1, 2002. The plaintiffs allege, among other things, fraud,
unjust enrichment and the violation of the federal Racketeer
Influenced and Corrupt Organizations Act (‘’RICO’’) and certain
state consumer fraud statutes and seek monetary and injunctive
relief, including treble damages.
Average Wholesale Price Litigation
A number of states as well as most counties in New York have sued
Pharmacia, Pfizer and other pharmaceutical manufacturers
alleging that they provided average wholesale price (AWP)
information for certain of their products that was higher than the
actual prices at which those products were sold. The AWP is used
to determine reimbursement levels under Medicare Part B and
Medicaid and in many private-sector insurance policies and
medical plans. The plaintiffs claim that the alleged spread between
the AWPs at which purchasers were reimbursed and the actual
prices was promoted by the defendants as an incentive to purchase
certain of their products. In addition to suing on their own behalf,
many of the plaintiff states seek to recover on behalf of individual
Medicare Part B co-payors and private-sector insurance companies
and medical plans in their states. These various actions generally
assert fraud claims as well as claims under state deceptive trade
practice laws, and seek monetary and other relief, including civil
penalties and treble damages. Several of the suits also allege
that Pharmacia and/or Pfizer did not report to the states its best
price for certain products under the Medicaid program.
In addition, Pharmacia, Pfizer and other pharmaceutical
manufacturers are defendants in a number of purported class
action suits in various federal and state courts brought by
employee benefit plans and other third-party payors that assert
claims similar to those in the state and county actions. These