Pfizer 2006 Annual Report Download - page 44
Download and view the complete annual report
Please find page 44 of the 2006 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
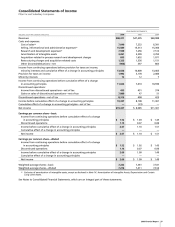
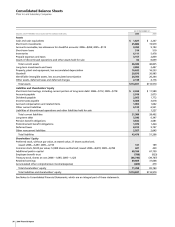
42 2006 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
reported in Cumulative effect of a change in accounting
principles—net of tax in the fourth quarter of 2005. In accordance
with these standards, we record accruals for legal obligations
associated with the retirement of tangible long-lived assets,
including obligations under the doctrine of promissory estoppel
and those that are conditional upon the occurrence of future
events. We recognize these obligations using management’s best
estimate of fair value.
As of January 1, 2004, we adopted the provisions of FIN 46R,
Consolidation of Variable Interest Entities (an interpretation of
ARB No. 51). FIN 46R provides additional guidance as to when
certain entities need to be consolidated for financial reporting
purposes. The adoption of FIN 46R did not have a material impact
on our consolidated financial statements.
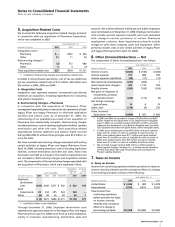
E. Acquisitions
Our consolidated financial statements and results of operations
reflect an acquired business after the completion of the acquisition
and are not restated. We account for acquired businesses using
the purchase method of accounting, which requires that the
assets acquired and the liabilities assumed be recorded at the date
of acquisition at their respective fair values. Any excess of the
purchase price over the estimated fair values of the net assets
acquired is recorded as goodwill. Amounts allocated to acquired
in-process research and development (IPR&D) are expensed at the
date of acquisition. When we acquire net assets that do not
constitute a business under GAAP, no goodwill is recognized.
F. Foreign Currency Translation
For most international operations, local currencies have been
determined to be the functional currencies. The effects of
converting non-functional currency assets and liabilities into the
functional currency are recorded in Other (income)/deductions—
net. We translate functional currency assets and liabilities to their
U.S. dollar equivalents at rates in effect at the balance sheet
date and record these translation adjustments in Shareholders’
equity—Accumulated other comprehensive income/(expense).
We translate functional currency statement of income amounts
at average rates for the period.
For operations in highly inflationary economies, we translate
monetary items at rates in effect at the balance sheet date, with
translation adjustments recorded in Other (income)/deductions—
net, and nonmonetary items at historical rates.
G. Revenues
Revenue Recognition—We record revenues from product sales
when the goods are shipped and title passes to the customer. At
the time of sale, we also record estimates for a variety of sales
deductions, such as sales rebates, discounts and incentives, and
product returns. When we cannot reasonably estimate the amount
of future product returns, we record revenues when the risk of
product return has been substantially eliminated.
Deductions from Revenues—Gross product sales are subject to a
variety of deductions that are generally estimated and recorded
in the same period that the revenues are recognized.
In the U.S., we record provisions for Medicaid, Medicare and
contract rebates based upon our actual experience ratio of rebates
paid and actual prescriptions during prior quarters. We apply
the experience ratio to the respective period’s sales to determine
the rebate accrual and related expense. This experience ratio is
evaluated regularly to ensure that the historical trends are as
current as practicable. As appropriate, we will adjust the ratio to
better match our current experience or our expected future
experience. In assessing this ratio, we consider current contract
terms, such as changes in formulary status and discount rates.
Our provisions for chargebacks (reimbursements to wholesalers
for honoring contracted prices to third parties) closely approximate
actual as we settle these deductions generally within two to
three weeks of incurring the liability.
Outside of the U.S., the majority of our rebates are contractual
or legislatively mandated and our estimates are based on actual
invoiced sales within each period; both of these elements help to
reduce the risk of variations in the estimation process. Some
European countries base their rebates on the government’s
unbudgeted pharmaceutical spending and we use an estimated
allocation factor based on historical payments against our actual
invoiced sales to project the expected level of reimbursement. We
obtain third-party information that helps us to monitor the
adequacy of these accruals.
We record sales allowances as a reduction of revenues at the time
the related revenues are recorded or when the allowance is offered,
whichever is later. We estimate the cost of our sales incentives based
on our historical experience with similar incentive programs.
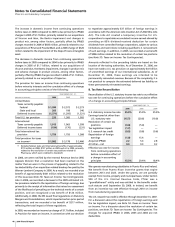
Our accruals for Medicaid rebates, Medicare rebates, performance-
based contract rebates and chargebacks were $1.5 billion as of
December 31, 2006, and $1.8 billion as of December 31, 2005.
Taxes collected from customers and remitted to governmental
authorities are presented on a net basis; that is, they are excluded
from revenues.
Alliances—We have agreements to co-promote pharmaceutical
products discovered by other companies. Revenues are earned
when our co-promotion partners ship the related product and title
passes to their customer. Alliance revenues are primarily based
upon a percentage of our co-promotion partners’ net sales.
Expenses for selling and marketing these products are included
in Selling, informational and administrative expenses.
H. Cost of Sales and Inventories
We value inventories at cost or fair value, if lower. Cost is
determined as follows:
•
finished goods and work in process at average actual cost; and
•
raw materials and supplies at average or latest actual cost.
I. Selling, Informational and Administrative Expenses
Selling, informational and administrative costs are expensed as
incurred. Among other things, these expenses include the costs
of marketing, advertising, shipping and handling, information
technology and non-plant employee compensation.
Advertising expenses relating to production costs are expensed
as incurred and the costs of radio time, television time and space
in publications are expensed when the related advertising occurs.
Advertising expenses totaled approximately $2.6 billion in 2006
and $2.7 billion in 2005 and 2004.