Pfizer 2006 Annual Report Download - page 19
Download and view the complete annual report
Please find page 19 of the 2006 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
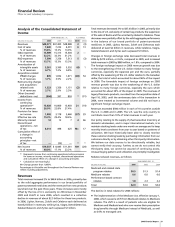
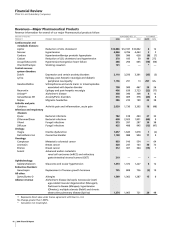
Pharmaceutical—Selected Product Descriptions
•
Lipitor, for the treatment of elevated LDL-cholesterol levels in
the blood, is the most widely used treatment for lowering
cholesterol and the best-selling pharmaceutical product of any
kind in the world, reaching about $12.9 billion in worldwide
sales in 2006, an increase of 6% compared to 2005. In the U.S.,
sales of $7.8 billion represent growth of 6% over 2005.
Internationally, Lipitor sales in 2006 increased 5% compared to
2005.
The growth in Lipitor revenues was driven by a combination of
factors, including dosage-form escalation and pricing (including
a favorable development in a pricing dispute in the U.S.), as well
as changes in rebate patterns. We continue to see aggressive
competition from branded and generic agents, particularly
when additional generic agents became available in the U.S.
near the end of 2006. Lipitor began to face competition in the
U.S. from generic pravastatin (Pravachol) in April 2006 and
generic simvastatin (Zocor) in June 2006, as well as other
competitive pressures. These launches have impacted the
dynamics of the statin market and increased pressure on Lipitor.
In October 2006, we launched a new advertising campaign
for Lipitor that highlights its strong benefit profile, particularly
its benefit in reducing the risk of heart attack and stroke in
patients with multiple risk factors for heart disease. This builds
on the consumer advertising that was implemented in April
2006. Scientific data continue to reinforce the trend toward the
use of higher dosages of statins for greater cholesterol
reduction.
See Notes to Consolidated Financial Statements—Note 19.
Legal Proceedings and Contingencies for a discussion of recent
developments with respect to certain patent litigation relating
to Lipitor.
•
Norvasc is the world’s most-prescribed branded medicine for
treating hypertension. Norvasc maintains exclusivity in many
major markets globally, including the U.S., Japan, Canada and
Australia, but has experienced patent expirations in many E.U.
countries. Norvasc sales in 2006 increased 3% compared to
2005. See Notes to Consolidated Financial Statements—Note 19.
Legal Proceedings and Contingencies for a discussion of recent
developments with respect to certain patent litigation relating
to Norvasc.
•
Caduet, single-pill therapy combining Norvasc and Lipitor,
recorded worldwide revenues of $370 million with a growth
rate of 99% in 2006 compared to 2005. Caduet was launched
in the U.S. in May 2004 and continues to grow at significantly
higher rates than the overall U.S. cardiovascular market. This
was largely driven by a more focused message platform and a
highly targeted consumer campaign. Caduet is available in
more than 15 other countries. Caduet has now received
approvals in 58 markets with drug applications pending in
nine additional markets and applications planned in 13 other
countries. In early 2007, Caduet is expected to be launched in
Spain and Taiwan.
See Notes to Consolidated Financial Statements—Note 19.
Legal Proceedings and Contingencies for a discussion of recent
developments with respect to certain patent litigation relating
to Caduet.
•
Chantix/Champix, the first new prescription treatment for
smoking cessation in nearly a decade, became available to
patients in the U.S. in August 2006. In September 2006, the
European Commission approved Champix in Europe for
smoking cessation and it was launched in select E.U. markets
in December 2006. Chantix/Champix is available with a patient
support plan, which smokers can customize to address their
individual behavioral triggers as they try to quit smoking. We
are pricing Chantix/Champix for a cash market, given the low
coverage for smoking-cessation products in medical plans.
•
Exubera, the first inhaled human insulin therapy for glycemic
control received approvals from both the FDA and the European
Commission for the treatment of adults with type 1 and type
2 diabetes in early 2006. Millions of people with diabetes are
not achieving or maintaining acceptable blood sugar levels,
despite the availability of current therapies. Exubera represents
a medical advance that offers to patients a novel method of
introducing insulin into their systems through the lungs. Since
May 2006, Exubera has been launched in Germany, Ireland, the
U.K. and in the U.S. Within the U.S., a comprehensive education
and training program for physicians was completed at the end
of 2006. During this time, we increased our understanding of
the fundamental drivers of the market. To further support
patients and healthcare professionals, Pfizer also provides a
24-hour-a-day, 7-day-a-week call center staffed by healthcare
professionals. Similar programs are also in place in European
markets where the product has been launched. An expanded
roll-out of Exubera to primary-care physicians in the U.S began
in January 2007. The manufacturing process for Exubera is
complex, involving novel technology. Initial supplies of Exubera
were available across the U.S. beginning in September 2006.
Sales to date have been minimal, reflecting a phased roll-out
of this product in connection with our education and training
programs for healthcare specialists.
•
Zoloft, which lost exclusivity in the U.S. in June 2006 and earlier
in many European markets, experienced a 35% revenue decline
in 2006 compared to 2005. It is indicated for the treatment of
major depressive disorder, panic disorder, obsessive-compulsive
disorder (OCD) in adults and children, post-traumatic stress
disorder (PTSD), premenstrual dysphoric disorder (PMDD) and
social anxiety disorder (SAD). Zoloft is approved for acute and
long-term use in all of these indications, with the exception of
PMDD. Zoloft was launched in Japan in July 2006 for the
indications of depression/depressed state and panic disorder.
•
Geodon/Zeldox, a psychotropic agent, is a dopamine and
serotonin receptor antagonist indicated for the treatment of
schizophrenia and acute manic or mixed episodes associated
with bipolar disorder. It is available in both an oral capsule and
rapid-acting intramuscular formulation. In the U.S., Geodon had
a new prescription share of 6.8% for December 2006. Geodon
has become the fastest growing anti-psychotic medication in
the U.S. In 2006, total Geodon worldwide sales grew 29%
compared to 2005. Geodon growth was driven by the
recognition of its efficacy by prescribers as clinical experience
increased, and by a favorable metabolic profile.
2006 Financial Report 17
Financial Review
Pfizer Inc and Subsidiary Companies