Pfizer 2006 Annual Report Download - page 25
Download and view the complete annual report
Please find page 25 of the 2006 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
R&D expense also includes payments for intellectual property
rights of $292 million in 2006, $156 million in 2005 and $160
million in 2004. (For further discussion, see the “Product
Developments” section of this Financial Review.)
Acquisition-Related In-Process Research and
Development Charges
The estimated value of acquisition-related IPR&D is expensed at
the acquisition date. In 2006, we expensed $835 million of IPR&D,
primarily related to our acquisitions of Rinat and PowderMed. In
2005, we expensed $1.7 billion of IPR&D, primarily related to
our acquisitions of Vicuron and Idun. In 2004, we expensed $1.1
billion of IPR&D, related primarily to our acquisition of Esperion.
Adapting to Scale Productivity Initiative
In connection with the AtS productivity initiative, which was
launched in early 2005 and broadened in October 2006, our
management has performed a comprehensive review of our
processes, organizations, systems and decision-making procedures
in a company-wide effort to improve performance and efficiency.
On January 22, 2007, we announced additional plans to
fundamentally change the way we run our business to meet the
challenges of a changing business environment and to take
advantage of the diverse opportunities in the marketplace. We
intend to generate cost savings through site rationalization in
research and manufacturing, streamlined organizational structures,
sales force and staff function reductions, and increased outsourcing
and procurement savings. Compared to 2006, we plan to achieve
a decrease in the SI&A pre-tax component of Adjusted income of
$500 million by the end of 2007, and an absolute net reduction of
the pre-tax expense component of Adjusted income of between
$1.5 billion and $2.0 billion by the end of 2008. (For an
understanding of Adjusted income, see the “Adjusted Income”
section of this Financial Review.) Savings realized during 2006
totaled approximately $2.6 billion. The actions associated with
the expanded AtS productivity initiative include restructuring
charges, such as asset impairments, exit costs and severance costs
(including any related impacts to our benefit plans, including
settlements and curtailments) and associated implementation
costs, such as accelerated depreciation charges, primarily associated
with plant network optimization efforts, and expenses associated
with system and process standardization and the expansion of
shared services (see Notes to Consolidated Financial Statements—
Note 4. Adapting to Scale Productivity Initiative).
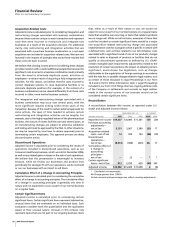
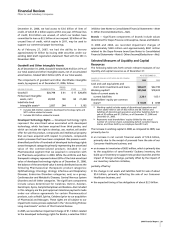
We incurred the following costs in connection with our AtS
productivity initiative:
YEAR ENDED DEC. 31,
_____________________________
(MILLIONS OF DOLLARS) 2006 2005
Implementation costs
(a)
$ 788 $325
Restructuring charges
(b)
1,296 438
Total AtS costs $2,084 $763
(a)
For 2006, included in Cost of sales ($392 million), Selling,
informational and administrative expenses ($243 million),
Research and development expenses ($176 million) and in Other
(income)/deductions—net ($23 million income). For 2005, included
in Cost of sales ($124 million), Selling, informational and
administrative expenses ($151 million), and Research and
development expenses ($50 million).
(b)
Included in Restructuring charges and acquisition-related costs.
Through December 31, 2006, the restructuring charges primarily
relate to our plant network optimization efforts and the
restructuring of our U.S. marketing and worldwide research and
development operations, and the implementation costs primarily
relate to system and process standardization, as well as the
expansion of shared services.
The components of restructuring charges associated with AtS follow:
UTILIZATION ACCRUAL
THROUGH AS OF
COSTS INCURRED
DEC. 31, DEC. 31,___________________________________
(MILLIONS OF DOLLARS) 2006 2005 TOTAL 2006 2006
(a)
Employee
termination
costs $ 809 $303 $1,112 $ 749 $363
Asset impairments
368 122 490 490 —
Other 119 13 132 93 39
$1,296 $438 $1,734 $1,332 $402
(a)
Included in Other current liabilities.
Through December 31, 2006, Employee termination costs
represent the approved reduction of the workforce by 8,274
employees, mainly in manufacturing, sales and research. We
notified affected individuals and 5,732 employees were terminated
as of December 31, 2006. Employee termination costs are recorded
as incurred and include accrued severance benefits, pension and
postretirement benefits. Asset impairments primarily include
charges to write down property, plant and equipment. Other
primarily includes costs to exit certain activities.
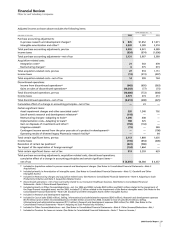
Acquisition-Related Costs
We incurred the following acquisition-related costs, primarily in
connection with our acquisition of Pharmacia on April 16, 2003:
YEAR ENDED DEC. 31,
__________________________________________
(MILLIONS OF DOLLARS) 2006 2005 2004
Integration costs
(a)
:
Pharmacia $ — $532 $ 454
Other 21 11 24
Restructuring charges
(a)
:
Pharmacia (3) 372 680
Other 9 3 (7)
Total acquisition-related costs $27 $918 $1,151
(a)
Included in Restructuring charges and acquisition-related costs.
In connection with the acquisition of Pharmacia, Pfizer
management approved plans to restructure and integrate the
operations of both legacy Pfizer and legacy Pharmacia to combine
operations, eliminate duplicative facilities and reduce costs. As of
December 31, 2005, the restructuring of our operations as a
result of our acquisition of Pharmacia was substantially complete.
Restructuring charges included severance, costs of vacating
duplicative facilities, contract termination and other exit costs.
Total acquisition-related expenditures (income statement and
balance sheet) incurred during 2002 through 2006 to achieve
these synergies were $5.2 billion, on a pre-tax basis.
Cost synergies from the Pharmacia acquisition were $4.2 billion
in 2005 and $3.6 billion in 2004. Synergies come from a broad
range of sources, including a streamlined organization, reduced
operating expenses, and procurement savings.
2006 Financial Report 23
Financial Review
Pfizer Inc and Subsidiary Companies