Pfizer 2006 Annual Report Download - page 46
Download and view the complete annual report
Please find page 46 of the 2006 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
44 2006 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
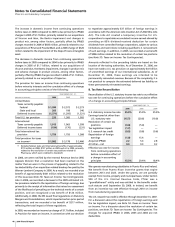
production technology, to manufacture and sell Exubera, an
inhaled form of insulin for use in adults with type 1 and type
2 diabetes, and the insulin-production business and facilities
located in Frankfurt, Germany, previously jointly owned by
Pfizer and sanofi-aventis, for approximately $1.4 billion in cash
(including transaction costs). In 2006, in connection with the
acquisition, as part of our final purchase price allocation, we
recorded $1.0 billion of developed technology rights, $218
million of inventory, and $166 million of Goodwill, all of which
have been allocated to our Pharmaceutical segment. The
amortization of the developed technology rights is primarily
included in Cost of sales. Prior to the acquisition, in connection
with our collaboration agreement with sanofi-aventis, we
recorded a research and development milestone due to us
from sanofi-aventis of $118 million ($71 million, after tax) in
Research and development expenses upon the approval of
Exubera in January 2006 by the FDA.
•
In December 2006, we completed the acquisition of PowderMed
Ltd. (PowderMed), a U.K. company which specializes in the
emerging science of DNA-based vaccines for the treatment of
influenza and chronic viral diseases, and in May 2006, we
completed the acquisition of Rinat Neurosciences Corp. (Rinat),
a biologics company with several new central-nervous-system
product candidates. In 2006, the aggregate cost of these and
other smaller acquisitions was approximately $880 million. In
connection with those transactions, we recorded $835 million in
Acquisition-related in-process research and development charges.
•
In September 2005, we completed the acquisition of all of the
outstanding shares of Vicuron Pharmaceuticals Inc. (Vicuron), a
biopharmaceutical company focused on the development of
novel anti-infectives, for approximately $1.9 billion in cash
(including transaction costs). In connection with the acquisition,
as part of our final purchase price allocation, we recorded $1.4
billion in Acquisition-related in-process research and development
charges, and $243 million of Goodwill, which has been allocated
to our Pharmaceutical segment.
•
In April 2005, we completed the acquisition of Idun
Pharmaceuticals Inc. (Idun), a biopharmaceutical company
focused on the discovery and development of therapies to
control apoptosis, and in August 2005, we completed the
acquisition of Bioren Inc. (Bioren), which focuses on technology
for optimizing antibodies. In 2005, the aggregate cost of these
and other smaller acquisitions was approximately $340 million
in cash (including transaction costs). In connection with these
transactions, we recorded $262 million in Acquisition-related in-
process research and development charges.
•
In September 2004, we completed the acquisition of
Campto/Camptosar (irinotecan), from sanofi-aventis for $525
million in cash (including transaction costs). In 2004, in
connection with the acquisition, as part of our final purchase
price allocation, we recorded $445 million of developed
technology rights, which have been allocated to our
Pharmaceutical segment.
•
In February 2004, we completed the acquisition of all the
outstanding shares of Esperion Therapeutics, Inc. (Esperion), a
biopharmaceutical company, for $1.3 billion in cash (including
transaction costs). In 2004, in connection with the acquisition,
as part of our final purchase price allocation, we recorded
$920 million in Acquisition-related in-process research and
development charges, and $239 million of Goodwill, which
has been allocated to our Pharmaceutical segment.
•
In 2004, we also completed several other small acquisitions. The
total purchase price associated with these transactions was
approximately $430 million in cash (including transaction costs).
In connection with these transactions, we recorded $151 million
in Acquisition-related in-process research and development
charges, and $206 million in intangible assets, primarily brands
(indefinite-lived) and developed technology rights, all of which
have been allocated to our Pharmaceutical segment.
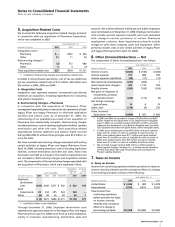
3. Discontinued Operations
We evaluate our businesses and product lines periodically for
strategic fit within our operations. As of December 31, 2006, we
sold the following:
•
In the fourth quarter of 2006, we sold our Consumer Healthcare
business for $16.6 billion, and recorded a gain of approximately
$10.2 billion ($7.9 billion, net of tax) in Gains on sales of
discontinued operations—net of tax in the consolidated
statement of income for 2006. This business was composed of:
substantially all of our former Consumer Healthcare segment;
other associated amounts, such as purchase-accounting
impacts, acquisition-related costs and restructuring and
implementation costs related to our Adapting to Scale (AtS)
productivity initiative that were previously reported in the
Corporate/Other segment; and
certain manufacturing facility assets and liabilities, which
were previously part of our Pharmaceutical or Corporate/
Other segment but were included in the sale of our Consumer
Healthcare business. The net impact to the Pharmaceutical
segment was not significant.
The results of this business are included in Income from
discontinued operations—net of tax for all periods presented.
Legal title to certain assets and legal control of the business in
certain non-U.S. jurisdictions did not transfer to the buyer on the
closing date of December 20 because the satisfaction of specific
local requirements was pending. These operations represent a
small portion of our Consumer Healthcare business and all are
expected to close within one year of the transaction date, most
within a few months. In order to ensure that the buyer was
placed in the same economic position as if the assets, operations
and activities of those businesses had been transferred on that
date, we entered into an agreement that passed the risks and
rewards of ownership to the buyer from December 20. We have
treated these delayed-close businesses as sold for accounting
purposes.
For a period of time, we will continue to generate cash flows
and to report income statement activity in Discontinued
operations—net of tax that are associated with our former
Consumer Healthcare business. The activities that will give rise
to these impacts are transitional in nature and generally result
from agreements that ensure and facilitate the orderly transfer