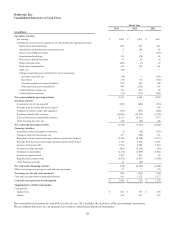
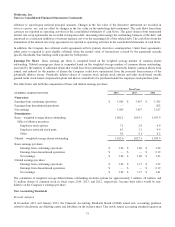
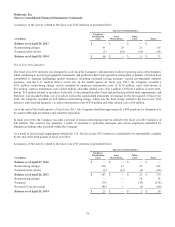
Medtronic 2014 Annual Report Download - page 79
Download and view the complete annual report
Please find page 79 of the 2014 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.Medtronic, Inc.
Notes to Consolidated Financial Statements (Continued)
Other Intangible Assets Other intangible assets include patents, trademarks, purchased technology, and IPR&D (since
April 25, 2009). Intangible assets with a definite life are amortized on a straight-line or accelerated basis, as appropriate, with
estimated useful lives ranging from three to 20 years. Intangible assets with a definite life are tested for impairment whenever
events or changes in circumstances indicate that the carrying amount of an intangible asset (asset group) may not be
recoverable. Indefinite-lived intangible assets are tested for impairment annually in the third quarter and whenever events or
changes in circumstances indicate that the carrying amount may be impaired. Impairment is calculated as the excess of the
asset’s carrying value over its fair value. Fair value is generally determined using a discounted future cash flow analysis.
IPR&D During fiscal year 2010, the Company adopted authoritative guidance related to business combinations. Subsequent
to the adoption of this guidance, IPR&D acquired in a business combination is capitalized at its fair value as an indefinite-lived
intangible asset. Prior to the adoption of this guidance, IPR&D was immediately expensed. The adoption of the authoritative
guidance did not change the requirement to expense IPR&D immediately with respect to asset acquisitions. IPR&D charges are
included within acquisition-related items in the consolidated statements of earnings. IPR&D has an indefinite life and is not
amortized until completion and development of the project, at which time the IPR&D becomes an amortizable asset. If the
related project is not completed in a timely manner or the project is terminated or abandoned, the Company may have an
impairment related to the IPR&D, calculated as the excess of the asset’s carrying value over its fair value.
The Company’s policy defines IPR&D as the fair value of those projects for which the related products have not received
regulatory approval and have no alternative future use. Determining the fair value of IPR&D acquired as part of a business
combination requires the Company to make significant estimates. The fair value assigned to IPR&D is determined by estimating
the future cash flows of each project or technology and discounting the net cash flows back to their present values. The discount
rate used is determined at the time of measurement in accordance with accepted valuation methodologies. These methodologies
include consideration of the risk of the project not achieving commercial feasibility.
At the time of acquisition, the Company expects that all acquired IPR&D will reach technological feasibility, but there can be
no assurance that the commercial viability of these products will actually be achieved. The nature of the efforts to develop the
acquired technologies into commercially viable products consists principally of planning, designing, and conducting clinical
trials necessary to obtain regulatory approvals. The risks associated with achieving commercialization include, but are not
limited to, delay or failure to obtain regulatory approvals to conduct clinical trials, delay or failure to obtain required market
clearances, or delays or issues with patent issuance, or validity and litigation. If commercial viability were not achieved, the
Company would likely look to other alternatives to provide these therapies.
Contingent Consideration During fiscal year 2010, as mentioned above, the Company adopted authoritative guidance related
to business combinations. Under this guidance, the Company must recognize contingent consideration at fair value at the
acquisition date. Prior to the adoption of this guidance, contingent consideration was not included on the balance sheet and was
recorded as incurred. The acquisition date fair value is measured based on the consideration expected to be transferred
(probability-weighted), discounted back to present value. The discount rate used is determined at the time of measurement in
accordance with accepted valuation methodologies. The fair value of the contingent consideration is remeasured at the estimated
fair value at each reporting period with the change in fair value recognized as income or expense within acquisition-related
items in the consolidated statements of earnings. Therefore, any changes in the fair value will impact the Company’s earnings in
such reporting period thereby resulting in potential variability in the Company’s earnings until contingencies are resolved.
Warranty Obligation The Company offers a warranty on various products. The Company estimates the costs that may be
incurred under its warranties and records a liability in the amount of such costs at the time the product is sold. Factors that affect
the Company’s warranty liability include the number of units sold, historical and anticipated rates of warranty claims, and cost
per claim. The Company periodically assesses the adequacy of its recorded warranty liabilities and adjusts the amounts as
necessary. The amount of the reserve recorded is equal to the net costs to repair or otherwise satisfy the claim. The Company
includes the warranty obligation in other accrued expenses and other long-term liabilities on the Company’s consolidated
balance sheets. The Company includes the covered costs associated with field actions, if any, in cost of products sold in the
Company’s consolidated statements of earnings.
71