Medtronic 2014 Annual Report Download - page 133
Download and view the complete annual report
Please find page 133 of the 2014 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Medtronic, Inc.
Notes to Consolidated Financial Statements (Continued)
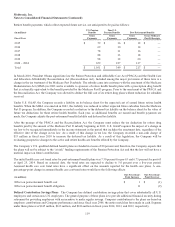
$10 million in fiscal year 2014, related to probable and reasonably estimated damages. In May 2014, the Company settled this
matter for $10 million and certain legal fees.
On October 14, 2010, the Company received a subpoena issued by the U.S. Attorney’s Office for the Western District of New
York pursuant to the Health Insurance Portability & Accountability Act of 1996, relating to the Company’s sales, marketing,
and reimbursement support practices regarding certain neurostimulation devices. The Company is fully cooperating with this
inquiry.
On November 9, 2010, the French Competition Authority commenced an investigation of the Company, along with a number of
other medical device companies, and the companies’ trade association, Syndicat National de l’Industrie des Technologies
Medicales (SNITEM), to determine whether such companies or SNITEM engaged in any anticompetitive practices in
responding to tenders to purchase certain medical devices. The Company is fully cooperating with the investigation.
On December 3, 2013, the Company received a subpoena for records from the U.S. Attorney’s Office for the District of
Minnesota related to the same topic addressed in its letter of May 6, 2013, requesting information relating to the Company’s
compliance with the Trade Agreements Act. The Company is fully cooperating with this inquiry.
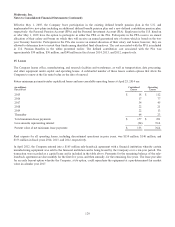
Except as described above, the Company has not recorded an expense related to losses in connection with these matters because
any potential loss is not currently probable or reasonably estimable under U.S. GAAP. Additionally, the Company cannot
reasonably estimate the range of loss, if any, that may result from these matters.
In the normal course of business, the Company periodically enters into agreements that require it to indemnify customers or
suppliers for specific risks, such as claims for injury or property damage arising out of the Company’s products or the
negligence of its personnel or claims alleging that its products infringe third-party patents or other intellectual property. The
Company’s maximum exposure under these indemnification provisions cannot be estimated, and the Company has not accrued
any liabilities within the consolidated financial statements. Historically, the Company has not experienced significant losses on
these types of indemnifications.
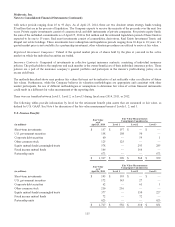
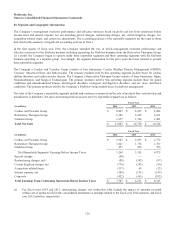
19. Quarterly Financial Data (unaudited)
(in millions,
except per share data) First Quarter Second Quarter Third Quarter Fourth Quarter Fiscal Year
Net Sales
2014 $ 4,083 $ 4,194 $ 4,163 $ 4,566 $ 17,005
2013 4,008 4,095 4,027 4,459 16,590
Gross Profit
2014 $ 3,061 $ 3,104 $ 3,113 $ 3,395 $ 12,672
2013 3,035 3,075 3,028 3,325 12,464
Net Earnings
2014 $ 953 $ 902 $ 762 $ 448 $ 3,065
2013 864 646 988 969 3,467
Basic Earnings per Share
2014 $ 0.94 $ 0.90 $ 0.76 $ 0.45 3.06
2013 0.84 0.63 0.98 0.96 3.40
Diluted Earnings per Share
2014 $ 0.93 $ 0.89 $ 0.75 $ 0.44 3.02
2013 0.83 0.63 0.97 0.95 3.37
The data in the schedule above has been intentionally rounded to the nearest million, and therefore, the quarterly amounts may
not sum to the fiscal year-to-date amounts.
125