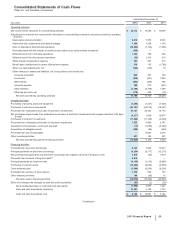
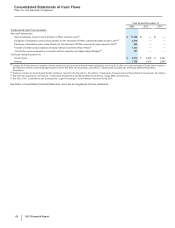
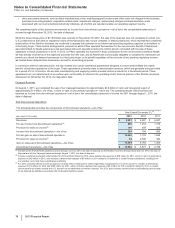
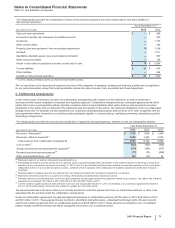
Pfizer 2013 Annual Report Download - page 65
Download and view the complete annual report
Please find page 65 of the 2013 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
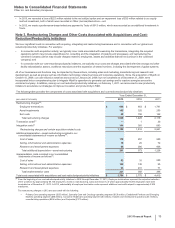
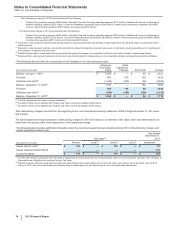
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
64
2013 Financial Report
I. Selling, Informational and Administrative Expenses
Selling, informational and administrative costs are expensed as incurred. Among other things, these expenses include the internal and
external costs of marketing, advertising, shipping and handling, information technology and legal defense.
Advertising expenses totaled approximately $3.0 billion in 2013, $2.8 billion in 2012 and $3.6 billion in 2011. Production costs are expensed as
incurred and the costs of radio time, television time and space in publications are expensed when the related advertising occurs.
J. Research and Development Expenses
Research and development (R&D) costs are expensed as incurred. These expenses include the costs of our proprietary R&D efforts, as well
as costs incurred in connection with certain licensing arrangements. Before a compound receives regulatory approval, we record upfront and
milestone payments made by us to third parties under licensing arrangements as expense. Upfront payments are recorded when incurred, and
milestone payments are recorded when the specific milestone has been achieved. Once a compound receives regulatory approval, we record
any milestone payments in Identifiable intangible assets, less accumulated amortization and, unless the asset is determined to have an
indefinite life, we amortize the payments on a straight-line basis over the remaining agreement term or the expected product life cycle,
whichever is shorter.
Research and development expenses related to upfront and milestone payments for intellectual property rights totaled $203 million in 2013,
$371 million in 2012 and $306 million in 2011.
K. Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets
Long-lived assets include:
• Goodwill—Goodwill represents the excess of the consideration transferred for an acquired business over the assigned values of its net
assets. Goodwill is not amortized.
• Identifiable intangible assets, less accumulated amortization—These acquired assets are recorded at cost. Intangible assets with finite
lives are amortized on a straight-line basis over their estimated useful lives. Intangible assets with indefinite lives that are associated with
marketed products are not amortized until a useful life can be determined. Intangible assets associated with IPR&D projects are not
amortized until approval is obtained in a major market, typically either the U.S. or the European Union (EU), or in a series of other
countries, subject to certain specified conditions and management judgment. The useful life of an amortizing asset generally is
determined by identifying the period in which substantially all of the cash flows are expected to be generated.
• Property, plant and equipment, less accumulated depreciation—These assets are recorded at cost and are increased by the cost of any
significant improvements after purchase. Property, plant and equipment assets, other than land and construction in progress, are
depreciated on a straight-line basis over the estimated useful life of the individual assets. Depreciation begins when the asset is ready for
its intended use. For tax purposes, accelerated depreciation methods are used as allowed by tax laws.
Amortization expense related to finite-lived acquired intangible assets that contribute to our ability to sell, manufacture, research, market and
distribute products, compounds and intellectual property is included in Amortization of intangible assets as these intangible assets benefit
multiple business functions. Amortization expense related to intangible assets that are associated with a single function and depreciation of
property, plant and equipment are included in Cost of sales, Selling, informational and administrative expenses and Research and
development expenses, as appropriate.
We review all of our long-lived assets for impairment indicators throughout the year and we perform detailed testing whenever impairment
indicators are present. In addition, we perform impairment testing for goodwill and indefinite-lived assets at least annually. When necessary,
we record charges for impairments.
Specifically:
• For finite-lived intangible assets, such as developed technology rights, and for other long-lived assets, such as property, plant and
equipment, whenever impairment indicators are present, we calculate the undiscounted value of the projected cash flows associated with
the asset, or asset group, and compare this estimated amount to the carrying amount. If the carrying amount is found to be greater, we
record an impairment loss for the excess of book value over fair value. In addition, in all cases of an impairment review, we re-evaluate
the remaining useful lives of the assets and modify them, as appropriate.
• For indefinite-lived intangible assets, such as Brands and IPR&D assets, when necessary, we determine the fair value of the asset and
record an impairment loss, if any, for the excess of book value over fair value. In addition, in all cases of an impairment review other than
for IPR&D assets, we re-evaluate whether continuing to characterize the asset as indefinite-lived is appropriate.
• For goodwill, when necessary, we determine the fair value of each reporting unit and compare that value to its book value. If the carrying
amount is found to be greater, we then determine the implied fair value of goodwill by subtracting the fair value of all the identifiable net
assets other than goodwill from the fair value of the reporting unit and record an impairment loss, if any, for the excess of the book value
of goodwill over the implied fair value.
Impairment reviews can involve a complex series of judgments about future events and uncertainties and can rely heavily on estimates and
assumptions. For information about the risks associated with estimates and assumptions, see Note 1C. Basis of Presentation and Significant
Accounting Policies: Estimates and Assumptions.