Pfizer 2013 Annual Report Download - page 29
Download and view the complete annual report
Please find page 29 of the 2013 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
28
2013 Financial Report
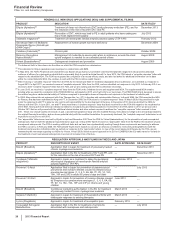
PENDING U.S. NEW DRUG APPLICATIONS (NDA) AND SUPPLEMENTAL FILINGS
PRODUCT INDICATION DATE FILED*
Eliquis (Apixaban)(a) Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and for
the reduction in the risk of recurrent DVT and PE
December 2013
Eliquis (Apixaban)(a) Prevention of DVT, which may lead to PE in adult patients who have undergone
hip or knee replacement surgery
July 2013
Tafamidis meglumine(b) Treatment of transthyretin familial amyloid polyneuropathy (TTR-FAP) February 2012
Genotropin Mark VII Multidose
Disposable Device (Somatropin
rDNA Origin)(c)
Replacement of human growth hormone deficiency December 2009
Celebrex (Celecoxib)(d) Chronic pain October 2009
Remoxy (Oxycodone
Hydrochloride)(e) Management of moderate-to-severe pain when a continuous, around-the-clock
opioid analgesic is needed for an extended period of time
August 2008
Viviant (Bazedoxifene)(f) Osteoporosis treatment and prevention August 2006
* The dates set forth in this column are the dates on which the FDA accepted our submissions.
(a) This indication for Eliquis (apixaban) was developed in collaboration with BMS.
(b) In May 2012, the FDA's Peripheral and Central Nervous System Drugs Advisory Committee voted that the tafamidis meglumine data provide substantial
evidence of efficacy for a surrogate endpoint that is reasonably likely to predict a clinical benefit. In June 2012, the FDA issued a “complete response” letter with
respect to the tafamidis NDA. The FDA has requested the completion of a second efficacy study, and also has asked for additional information on the data
within the current tafamidis NDA. We continue to work with the FDA to define a path forward.
(c) After receiving a “complete response” letter from the FDA for the Genotropin Mark VII multidose disposable device submission, we submitted our response in
August 2010. In April 2011, we received a second “complete response” letter from the FDA, and we submitted our response in July 2013. In February 2014, we
received a third "complete response" letter from the FDA, and we are working with the FDA to determine next steps.
(d) In June 2010, we received a “complete response” letter from the FDA for the Celebrex chronic pain supplemental NDA. The supplemental NDA remains
pending while we await the completion of the PRECISION trial, anticipated in 2015, which will inform our next steps. The PRECISION trial is designed to assess
the relative long-term cardiovascular safety of Celebrex compared to prescription doses of ibuprofen and naproxen in the treatment of arthritis pain.
(e) In 2005, King entered into an agreement with Pain Therapeutics, Inc. (PT) to develop and commercialize Remoxy. In August 2008, the FDA accepted the NDA
for Remoxy that had been submitted by King and PT. In December 2008, the FDA issued a “complete response” letter. In March 2009, King exercised its right
under the agreement with PT to assume sole control and responsibility for the development of Remoxy. In December 2010, King resubmitted the NDA for
Remoxy with the FDA. In June 2011, we and PT announced that a “complete response” letter had been received from the FDA with regard to the resubmission
of the NDA. Having achieved technical milestones related to manufacturing and following guidance received from the FDA earlier in 2013, we announced in
October 2013 that we will proceed with the additional clinical studies and other actions required to address the "complete response" letter received in June
2011. These new clinical studies will include, in part, a pivotal bioequivalence study with the modified Remoxy formulation to bridge to the clinical data related to
the original Remoxy formulation, and an abuse-potential study with the modified formulation. As previously disclosed, the "complete response" submission is not
expected to occur prior to mid-2015.
(f) Two “approvable” letters were received by Wyeth in April and December 2007 from the FDA for Viviant (bazedoxifene), for the prevention of post-menopausal
osteoporosis, that set forth the additional requirements for approval. In May 2008, Wyeth received an “approvable” letter from the FDA for the treatment of post-
menopausal osteoporosis. The FDA is seeking additional data, and we have been systematically working through these requirements and seeking to address
the FDA's concerns. In February 2008, the FDA advised Wyeth that it expects to convene an advisory committee to review the pending NDAs for both the
treatment and prevention indications after we submit our response to the “approvable” letters. In view of the recent approval of Duavee by the FDA, we are
reassessing the next steps regarding our NDAs for Viviant. In April 2009, Wyeth received approval in the EU for CONBRIZA (the EU trade name for Viviant) for
the treatment of post-menopausal osteoporosis in women at increased risk of fracture.
REGULATORY APPROVALS AND FILINGS IN THE EU AND JAPAN
PRODUCT DESCRIPTION OF EVENT DATE APPROVED DATE FILED*
Bosulif (Bosutinib) Application filed in Japan for treatment of previously treated
chronic myelogenous leukemia
— December 2013
Eliquis (Apixaban)(a) Application filed in the EU for treatment of DVT and PE, and
for the reduction in the risk of recurrent DVT and PE
— November 2013
Vyndaqel (Tafamidis
meglumine)
Approval in Japan as a treatment to delay the peripheral
neurological impairment of transthyretin familial amyloid
polyneuropathy (TTR-FAP)
September 2013 —
Prevenar 13 Adult Application filed in Japan for prevention of pneumococcal
pneumonia and invasive disease caused by Streptococcus
pneumoniae serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C,
19A, 19F and 23F) in adults 65 years of age and older
— July 2013
Prevenar 13 Infant Approval in Japan for prevention of invasive disease caused
by Streptococcus pneumoniae serotypes (1, 3, 4, 5, 6A, 6B,
7F, 9V, 14, 18C, 19A, 19F and 23F) in infants and young
children
June 2013 __
Bosulif (Bosutinib) Conditional marketing authorization in the EU for treatment
of previously treated chronic myelogenous leukemia
March 2013 —
Xeljanz (Tofacitinib) Approval in Japan for treatment of rheumatoid arthritis with
inadequate response to existing therapies
March 2013 —
Lyrica (Pregabalin) Approval in Japan for treatment of neuropathic pain February 2013 —
Conjugated Estrogens/
Bazedoxifene
Application filed in the EU for treatment of symptoms
associated with menopause and osteoporosis
— July 2012