Pfizer 2013 Annual Report Download - page 48
Download and view the complete annual report
Please find page 48 of the 2013 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
2013 Financial Report
47
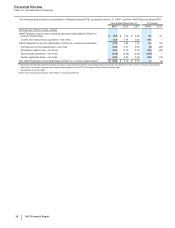
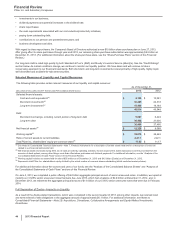
Dividends on Common Stock
We paid dividends on our common stock of $6.6 billion in 2013 and $6.5 billion in 2012. In December 2013, our Board of Directors declared a
first-quarter 2014 dividend of $0.26 per share, payable on March 4, 2014, to shareholders of record at the close of business on February 7,
2014. The first-quarter 2014 cash dividend will be our 301st consecutive quarterly dividend.
Our current and projected dividends provide a return to shareholders while maintaining sufficient capital to invest in growing our businesses
and to seek to increase shareholder value. Our dividends are not restricted by debt covenants. While the dividend level remains a decision of
Pfizer’s Board of Directors and will continue to be evaluated in the context of future business performance, we currently believe that we can
support future annual dividend increases, barring significant unforeseen events.
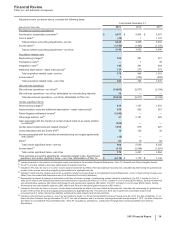
NEW ACCOUNTING STANDARDS
Recently Adopted Accounting Standard
See Notes to Consolidated Financial Statements—Note 1B. Basis of Presentation and Significant Accounting Policies: Adoption of New
Accounting Standard.
Recently Issued Accounting Standards, Not Adopted as of December 31, 2013
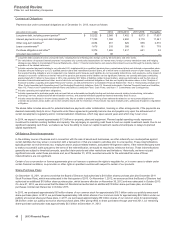
In March 2013, the Financial Accounting Standards Board (FASB) issued a clarification regarding the accounting for cumulative translation
adjustment (CTA) upon derecognition of assets or investment within a foreign entity. This new standard provides additional CTA accounting
guidance on sales or transfers of foreign entity investments and assets as well as step acquisitions involving a foreign entity. The provisions of
the new standard are effective on a prospective basis in 2014 for annual and interim reporting periods. We do not expect the provisions of this
standard to have a significant impact on our consolidated financial statements.
In February 2013, the FASB issued guidance regarding the measurement of obligations resulting from joint and several liability arrangements
that may include debt agreements, other contractual obligations and settled litigation or judicial rulings. The provisions of this standard require
that these obligations are measured at the amount representing the agreed-upon obligation of the company as well as additional liability
amounts it expects to assume on behalf of other parties in the arrangement. The provisions of the new standard are effective on a
retrospective basis in 2014 for annual and interim reporting periods. We do not expect the provisions of this standard to have a significant
impact on our consolidated financial statements.
FORWARD-LOOKING INFORMATION AND FACTORS THAT MAY AFFECT FUTURE RESULTS
This report and other written or oral statements that we make from time to time contain forward-looking statements that set forth anticipated
results based on management’s plans and assumptions. Such forward-looking statements involve substantial risks and uncertainties. We have
tried, wherever possible, to identify such statements by using words such as “will,” “anticipate,” “estimate,” “expect,” “project,” “intend,” “plan,”
“believe,” “target,” “forecast,” “goal”, “objective”, "aim" and other words and terms of similar meaning or by using future dates in connection
with any discussion of, among other things, our anticipated future operating or financial performance, business plans and prospects, in-line
products and product candidates, strategic reviews, capital allocation, business-development plans and plans relating to share repurchases
and dividends. In particular, these include statements relating to future actions, business plans and prospects, prospective products or product
approvals, future performance or results of current and anticipated products, sales efforts, expenses, interest rates, foreign exchange rates,
the outcome of contingencies, such as legal proceedings, plans relating to share repurchases and dividends, government regulation and
financial results, including, in particular, the financial guidance set forth in the “Our Financial Guidance for 2014” section of this Financial
Review, the anticipated costs and cost savings set forth in the “Restructuring Charges and Other Costs Associated with Acquisitions and Cost-
Reduction/Productivity Initiatives” section of this Financial Review, the planned capital spending set forth in the "Contractual Obligations"
section of this Financial Review, and the contributions that we expect to make from our general assets to the Company's pension and
postretirement plans during 2014 set forth in the "Contractual Obligations" section of this Financial Review and in Notes to Consolidated
Financial Statements––Note 11. Pension and Postretirement Benefit Plans and Defined Contribution Plans. Among the factors that could
cause actual results to differ materially from past results and future plans and projected future results are the following:
• the outcome of research and development activities including, without limitation, the ability to meet anticipated clinical trial
commencement and completion dates, regulatory submission and approval dates, and launch dates for product candidates, as
well as the possibility of unfavorable clinical trial results, including unfavorable new clinical data and additional analyses of
existing clinical data;
• decisions by regulatory authorities regarding whether and when to approve our drug applications, as well as their decisions
regarding labeling, ingredients and other matters that could affect the availability or commercial potential of our products;
• the speed with which regulatory authorizations, pricing approvals and product launches may be achieved;
• the outcome of post-approval clinical trials, which could result in the loss of marketing approval for a product or changes in the
labeling for, and/or increased or new concerns about the safety or efficacy of, a product that could affect its availability or
commercial potential;
• the success of external business-development activities;
• competitive developments, including the impact on our competitive position of new product entrants, in-line branded products,
generic products, private label products and product candidates that treat diseases and conditions similar to those treated by
our in-line drugs and drug candidates;