Pfizer 2008 Annual Report Download - page 61
Download and view the complete annual report
Please find page 61 of the 2008 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
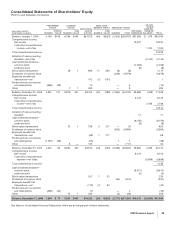
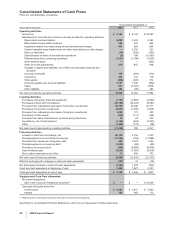
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
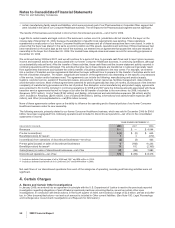
amounts associated with uncertain tax positions of approximately $4.0 billion, including the associated accrued interest of
approximately $780 million, from current to noncurrent. (See Note 7E. Taxes on Income: Tax Contingencies.)
B. Taxes on Income
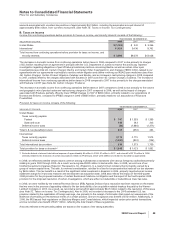
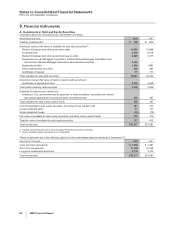
Income from continuing operations before provision for taxes on income, and minority interests consists of the following:
YEAR ENDED DECEMBER 31,
(MILLIONS OF DOLLARS) 2008 2007 2006
United States $ (1,760) $ 242 $ 3,266
International 11,454 9,036 9,762
Total income from continuing operations before provision for taxes on income, and
minority interests $ 9,694 $9,278 $13,028
The decrease in domestic income from continuing operations before taxes in 2008 compared to 2007 is due primarily to charges
of $2.3 billion resulting from an agreement in principle with the U.S. Department of Justice to resolve the previously reported
investigation regarding allegations of past off-label promotional practices concerning Bextra, as well as certain other open
investigations (see Note 4A.Certain Charges: Bextra and Certain Other Investigations), and charges of $900 million related to our
agreements and our agreements in principle to resolve certain litigation and claims involving our NSAID pain medicines (see Note
4B.Certain Charges: Certain Product Litigation–Celebrex and Bextra), and an increase in restructuring charges in 2008 compared
to 2007, partially offset by the charges associated with Exubera in 2007 (see Note 4D. Certain Charges: Exubera). The increase in
international income from continuing operations before taxes in 2008 compared to 2007 is due primarily to the charges associated
with Exubera in 2007 (see Note 4D. Certain Charges: Exubera).
The decrease in domestic income from continuing operations before taxes in 2007 compared to 2006 is due primarily to the volume
and geographic mix of product sales and restructuring charges in 2007 compared to 2006, as well as the impact of charges
associated with Exubera, partially offset by lower IPR&D charges in 2007 of $283 million, primarily related to our acquisitions of
Biorexis and Embrex, compared to IPR&D charges in 2006 of $835 million, primarily related to our acquisitions of Rinat and
PowderMed.
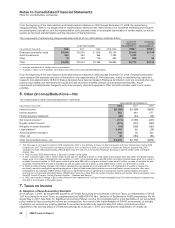
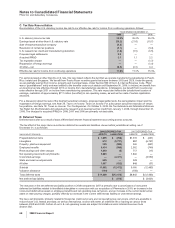
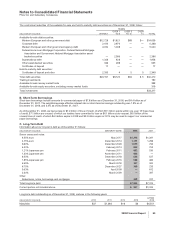
Provision for taxes on income consists of the following:
YEAR ENDED DECEMBER 31,
(MILLIONS OF DOLLARS) 2008 2007 2006
United States:
Taxes currently payable:
Federal $ 707 $ 1,393 $ 1,399
State and local 154 243 205
Deferred income taxes (30) (1,986) (1,371)
Total U.S. tax (benefit)/provision 831 (350) 233
International:
Taxes currently payable 2,115 2,175 1,913
Deferred income taxes (1,301) (802) (154)
Total international tax provision 814 1,373 1,759
Total provision for taxes on income(a) $ 1,645 $ 1,023 $ 1,992
(a) Excludes federal, state and international expense of approximately $4 million in 2008, $1 million in 2007, and a benefit of $119 million in 2006,
primarily related to the resolution of certain tax positions related to Pharmacia, which were debited or credited to Goodwill, as appropriate.
In 2008, we effectively settled certain issues common among multinational corporations with various foreign tax authorities primarily
relating to years 2000 through 2005. As a result, we recognized $305 million in tax benefits. Also, in 2008, we sold one of our
biopharmaceutical companies, Esperion Therapeutics, Inc. (Esperion), to a newly formed company that is majority-owned by a
group of venture capital firms. The sale, for nominal consideration, resulted in a loss for tax purposes that reduced our tax expense
by $426 million. This tax benefit is a result of the significant initial investment in Esperion in 2004, primarily reported as an income
statement charge for in-process research and development at acquisition date. 2008 also reflects the impact of the third-quarter
2008 provision for the proposed resolution of certain Bextra and Celebrex civil litigation and the impact of the fourth-quarter 2008
provision for the proposed resolution of certain investigations, which are either not deductible or deductible at lower tax rates.
In 2006, we were notified by the Internal Revenue Service (IRS) Appeals Division that a resolution had been reached on the matter
that we were in the process of appealing related to the tax deductibility of an acquisition-related breakup fee paid by the Warner-
Lambert Company in 2000. As a result, we recorded a tax benefit of approximately $441 million related to the resolution of this issue
(see Note 7E. Taxes on Income: Tax Contingencies). Also in 2006, we recorded a decrease to the 2005 estimated U.S. tax
provision related to the repatriation of foreign earnings, due primarily to the receipt of information that raised our assessment of the
likelihood of prevailing on the technical merits of a certain position, and we recognized a tax benefit of $124 million. Additionally, in
2006, the IRS issued final regulations on Statutory Mergers and Consolidations, which impacted certain prior-period transactions,
and we recorded a tax benefit of $217 million, reflecting the total impact of these regulations.
Amounts reflected in the preceding tables are based on the location of the taxing authorities.
2008 Financial Report 59