Pfizer 2008 Annual Report Download - page 4
Download and view the complete annual report
Please find page 4 of the 2008 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc and Subsidiary Companies
Oan aggregate year-over-year increase in revenues from products launched since 2006; and
Othe solid aggregate performance of the balance of our broad portfolio of patent-protected medicines,
offset by:
Othe impact of loss of U.S. exclusivity on Norvasc in March 2007 and Camptosar in February 2008; and
Othe impact of loss of U.S. exclusivity and cessation of selling of Zyrtec/Zyrtec D in January 2008.
Norvasc, Camptosar and Zyrtec/Zyrtec D collectively experienced a decline in revenues of about $2.6 billion in 2008 compared to
2007. The significant product and alliance revenue impacts on revenues for 2008, compared to 2007, are as follows:
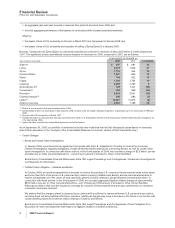
YEAR ENDED DECEMBER 31,
(MILLIONS OF DOLLARS) 2008 2007 % CHANGE
Sutent(a) $ 847 $ 581 46
Lyrica 2,573 1,829 41
Zyvox 1,115 944 18
Geodon/Zeldox 1,007 854 18
Vfend 743 632 18
Viagra 1,934 1,764 10
Celebrex 2,489 2,290 9
Zyrtec/Zyrtec D(b) 129 1,541 (92)
Camptosar(b) 563 969 (42)
Norvasc(c) 2,244 3,001 (25)
Chantix/Champix(d) 846 883 (4)
Lipitor(e) 12,401 12,675 (2)
Alliance revenues 2,251 1,789 26
(a) Sutent is a new product that was launched since 2006.
(b) Zyrtec/Zyrtec D lost U.S. exclusivity in late January 2008, at which time we ceased selling this product. Camptosar lost U.S. exclusivity in February
2008.
(c) Norvasc lost U.S. exclusivity in March 2007.
(d) Chantix/Champix is a new product that was launched since 2006. U.S. prescription trends and revenues have declined following the changes to its
U.S. label during 2008.
(e) Lipitor has been impacted by competitive pressures and other factors.
As of September 30, 2008, our portfolio of medicines included nine medicines that led their therapeutic areas based on revenues.
(See further discussion in the “Analysis of the Consolidated Statement of Income” section of this Financial Review.)
•Certain Charges
OBextra and Certain Other Investigations
In January 2009, we entered into an agreement in principle with the U.S. Department of Justice to resolve the previously
reported investigation regarding allegations of past off-label promotional practices concerning Bextra, as well as certain other
open investigations. In connection with these actions, in the fourth quarter of 2008, we recorded a charge of $2.3 billion, pre-tax
and after-tax, in Other (income)/deductions – net and such amount is included in Other current liabilities.
See Notes to Consolidated Financial Statements–Note 19D. Legal Proceedings and Contingencies: Government Investigations
and Requests for Information.
OCertain Product Litigation – Celebrex and Bextra
In October 2008, we reached agreements in principle to resolve the pending U.S. consumer fraud purported class action cases
and more than 90% of the known U.S. personal injury claims involving Celebrex and Bextra, and we reached agreements to
resolve substantially all of the claims of state attorneys general primarily relating to alleged Bextra promotional practices. In
connection with these actions, in the third quarter of 2008, we recorded aggregate litigation-related charges of approximately
$900 million, pre-tax, in Other (income)/deductions—net. Virtually all of this amount is included in Other current liabilities.
Although we believe that we have insurance coverage for a portion of the proposed personal injury settlements, no insurance
recoveries have been recorded.
We believe that the charges related to personal injury claims will be sufficient to resolve all known U.S. personal injury claims,
including those not being settled at this time. However, additional charges may have to be taken in the future in connection with
certain pending claims and unknown claims relating to Celebrex and Bextra.
See Notes to Consolidated Financial Statements–Note 19B. Legal Proceedings and Contingencies: Product Litigation for a
discussion of recent developments with respect to litigation related to Celebrex and Bextra.
2 2008 Financial Report