Pfizer 2008 Annual Report Download - page 11
Download and view the complete annual report
Please find page 11 of the 2008 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc and Subsidiary Companies
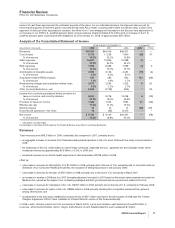
During 2008, we achieved a reduction of about $1.6 billion in the Selling, informational and administrative expenses (SI&A) pre-tax
component of Adjusted income compared to 2006, on a constant currency basis (the actual foreign exchange rates in effect in
2006). In 2008 and 2007, we achieved a total net reduction of the pre-tax total expense component of Adjusted income of $2.8
billion, compared to 2006 on a constant currency basis (the actual foreign exchange rates in effect in 2006). (For an understanding
of Adjusted income, see the “Adjusted Income” section of this Financial Review.) These cost reductions have been achieved despite
inflation and compensation increases over the period.
On January 26, 2009, we announced the implementation of a new cost-reduction initiative that we anticipate will achieve a reduction
in adjusted total costs of approximately $3 billion, based on the actual foreign exchange rates in effect during 2008, by the end of
2011, compared with our 2008 adjusted total costs. We expect that this program will be completed by the end of 2010, with full
savings to be realized by the end of 2011. We plan to reinvest approximately $1 billion of these savings in the business, resulting in
an expected $2 billion net decrease compared to our 2008 adjusted total costs. (For an understanding of Adjusted income, see the
“Adjusted income” section of this Financial Review.)
As part of this new cost-reduction initiative, we intend to reduce our total worldwide workforce by approximately 10%. Reductions will
span sales, manufacturing, research and development, and administrative organizations. We expect to incur costs related to this
new cost-reduction initiative of approximately $6 billion, pre-tax, of which $1.5 billion was recorded in 2008.
Projects in various stages of implementation include:
Pfizer Global Research and Development (PGRD)—
•Creating a More Agile and Productive Organization—In January 2009, we announced that we plan to reduce our global research staff.
We expect these reductions, which are part of the planned 10% total workforce reduction discussed above, will be completed during
2009.
After a review of all our therapeutic areas, in 2008, we announced our decision to exit certain disease areas—anemia,
atherosclerosis/hyperlipidemia, bone health/frailty, gastrointestinal, heart failure, liver fibrosis, muscle, obesity, osteoarthritis
(disease modifying concepts only) and peripheral arterial disease—and give higher priority to the following disease areas:
Alzheimer's disease, diabetes, inflammation/immunology, oncology, pain and psychoses (schizophrenia). We also will continue to
work in many other disease areas, such as asthma, chronic obstructive pulmonary disorder, genitourinary, infectious diseases,
ophthalmology, smoking cessation, thrombosis and transplant, among others. With a smaller, more focused research portfolio, we
will be able to devote our resources to the most valuable opportunities. These decisions did not affect our portfolio of marketed
products, the development of compounds currently in Phase 3 or any launches planned over the next three years.
In 2007, we consolidated each research therapeutic area into a single site and focused our research network by closing R&D
sites. Since then, we have ceased pharmaceutical R&D operations in six sites that were previously identified for exit by PGRD:
Mumbai, India; Plymouth Township, Michigan; Ann Arbor, Michigan; Kalamazoo, Michigan; Nagoya, Japan; and Amboise,
France. The facilities in Mumbai, Plymouth Township and downtown Kalamazoo have been disposed of. We are under contract
for sale of the entire Ann Arbor campus, with an anticipated closing in mid-2009. In mid-2008, the former Pfizer R&D site in
Nagoya became the base of operations of an R&D spin-off in which Pfizer retains a small interest. R&D operations in Amboise
have ceased and decommissioning of the R&D site is now underway.
We continue to focus on reduced cycle time and improved compound survival in the drug discovery and development process.
Notable cycle time improvements have been demonstrated in the period from Compound Selection to the start of Phase 1. In
addition, over the next two years, we expect to see a 25% to 33% reduction in cycle time in the period from Final Approved
Protocol to Last Subject-First Visit, as new processes and procedures are adopted for newly initiated Phase 2, 3 and 4 clinical
trials. In the past couple of years, a number of steps have been taken to improve compound survival, such as rigorous analyses of
the successful and unsuccessful projects in the entire portfolio to ensure that results are captured and applied to on-going
programs and to portfolio decisions.
Pfizer Global Manufacturing (PGM)—
•Supply Network Transformation—To ensure that our manufacturing facilities are aligned with current and future product needs, we are
continuing to optimize Pfizer’s network of plants. We have focused on innovation and delivering value through a simplified supply
network. Since 2005, 34 sites have been identified for rationalization. In addition, there have been extensive consolidations and
realignments of operations resulting in streamlined operations and staff reductions.
We are moving our global manufacturing network into a global strategic supply network, consisting of our internal network of
plants together with strategic external manufacturers, and including purchasing, packaging and distribution. As of the end of 2008,
we have reduced our internal network of plants from 93 five years ago to 46, which includes the acquisition of seven plants and
the sites sold in 2006 as part of our Consumer Healthcare business. We plan to reduce our internal network of plants around the
world to 41. We expect that the cumulative impact will be a more focused, streamlined and competitive manufacturing operation,
with less than 50% of our former internal plants and more than 48% fewer manufacturing employees, compared to 2003. As part
of our global strategic supply network, we currently expect to increase outsourced manufacturing of our products from
approximately 17% of our products, on a cost basis, to approximately 30% over the next two to three years.
Worldwide Pharmaceutical Operations (WPO)—
•Reorganization of our Field Force—As part of Pfizer’s overall restructuring into smaller, more focused business units, we have changed
our global field force operations to enable us to adapt to changing market dynamics and respond to local customer needs more quickly
2008 Financial Report 9