Pfizer 2008 Annual Report Download - page 42
Download and view the complete annual report
Please find page 42 of the 2008 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
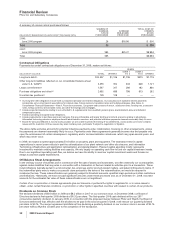
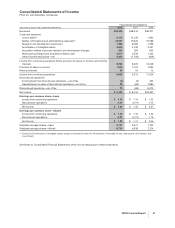
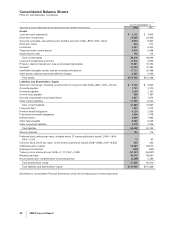
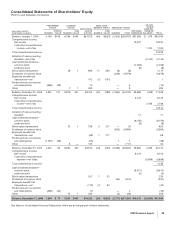
Financial Review
Pfizer Inc and Subsidiary Companies
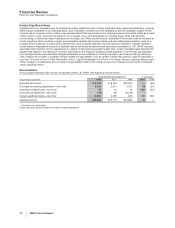
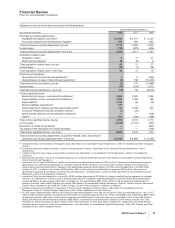
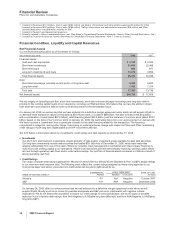
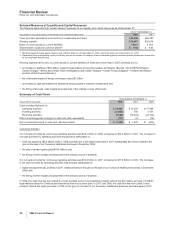
Forward-Looking Information and Factors That May Affect Future Results
The Securities and Exchange Commission encourages companies to disclose forward-looking information so that investors can
better understand a company’s future prospects and make informed investment decisions. This report and other written or oral
statements that we make from time to time contain such forward-looking statements that set forth anticipated results based on
management’s plans and assumptions. Such forward-looking statements involve substantial risks and uncertainties. We have tried,
wherever possible, to identify such statements by using words such as “will,” “anticipate,” “estimate,” “expect,” “project,” “intend,”
“plan,” “believe,” “target,” “forecast” and other words and terms of similar meaning in connection with any discussion of future
operating or financial performance or business plans and prospects. In particular, these include statements relating to future actions,
business plans and prospects, prospective products or product approvals, future performance or results of current and anticipated
products, sales efforts, expenses, interest rates, foreign exchange rates, the outcome of contingencies, such as legal proceedings,
and financial results. Among the factors that could cause actual results to differ materially are the following:
•Success of research and development activities;
•Decisions by regulatory authorities regarding whether and when to approve our drug applications as well as their decisions regarding
labeling and other matters that could affect the availability or commercial potential of our products;
•Speed with which regulatory authorizations, pricing approvals and product launches may be achieved;
•Success of external business-development activities;
•Competitive developments, including with respect to competitor drugs and drug candidates that treat diseases and conditions similar to
those treated by our in-line drugs and drug candidates;
•Ability to successfully market both new and existing products domestically and internationally;
•Difficulties or delays in manufacturing;
•Trade buying patterns;
•Ability to meet generic and branded competition after the loss of patent protection for our products and competitor products;
•Impact of existing and future legislation and regulatory provisions on product exclusivity;
•Trends toward managed care and healthcare cost containment;
•U.S. legislation or regulatory action affecting, among other things, pharmaceutical product pricing, reimbursement or access, including
under Medicaid, Medicare and other publicly funded or subsidized health programs; the importation of prescription drugs from outside
the U.S. at prices that are regulated by governments of various foreign countries; direct-to-consumer advertising and interactions with
healthcare professionals; and the use of comparative effectiveness methodologies that could be implemented in a manner that focuses
primarily on the cost differences and minimizes the therapeutic differences among pharmaceutical products and restricts access to
innovative medicines;
•Impact of the Medicare Prescription Drug, Improvement and Modernization Act of 2003;
•Legislation or regulatory action in markets outside the U.S. affecting pharmaceutical product pricing, reimbursement or access;
•Contingencies related to actual or alleged environmental contamination;
•Claims and concerns that may arise regarding the safety or efficacy of in-line products and product candidates;
•Significant breakdown, infiltration or interruption of our information technology systems and infrastructure;
•Legal defense costs, insurance expenses, settlement costs and the risk of an adverse decision or settlement related to product liability,
patent protection, governmental investigations, ongoing efforts to explore various means for resolving asbestos litigation, and other
legal proceedings;
•Ability to protect our patents and other intellectual property both domestically and internationally;
•Interest rate and foreign currency exchange rate fluctuations;
•Governmental laws and regulations affecting domestic and foreign operations, including tax obligations;
•Changes in U.S. generally accepted accounting principles;
•Uncertainties related to general economic, political, business, industry, regulatory and market conditions including, without limitation,
uncertainties related to the impact on us, our lenders, our customers, our suppliers and counterparties to our foreign-exchange and
interest-rate agreements, of the global economic recession and recent and possible future changes in global financial markets;
•Any changes in business, political and economic conditions due to actual or threatened terrorist activity in the U.S. and other parts of
the world, and related U.S. military action overseas;
40 2008 Financial Report