Pfizer 2008 Annual Report Download - page 57
Download and view the complete annual report
Please find page 57 of the 2008 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
Q. Share-Based Payments
Our compensation programs can include share-based payments. All grants under share-based payment programs are accounted for
at fair value and these fair values are generally amortized on an even basis over the vesting terms into Cost of sales, Selling,
informational and administrative expenses and Research and development expenses, as appropriate.
2. Acquisitions
We are committed to capitalizing on new growth opportunities, a strategy that can include acquisitions of companies, products or
technologies. During the three years ended December 31, 2008, 2007 and 2006, we acquired the following:
•In the fourth quarter of 2008, we concluded the acquisition of a number of animal health product lines from Schering-Plough
Corporation (Schering-Plough) for sale in the European Economic Area in the following categories: swine e.coli vaccines; equine
influenza and tetanus vaccines; ruminant neonatal and clostridia vaccines; rabies vaccines; companion animal veterinary specialty
products; and parasiticides and anti-inflammatories. The cost of acquiring these product lines was approximately $170 million.
•In the second quarter of 2008, we acquired Encysive Pharmaceuticals Inc. (Encysive), a biopharmaceutical company, whose main
product (Thelin), for the treatment of pulmonary arterial hypertension, is commercially available in much of the E.U., is approved in
certain other markets, and is under review by the U.S. Food and Drug Administration (FDA). The cost of acquiring Encysive, through a
tender offer and subsequent merger, was approximately $200 million, including transaction costs. Upon our acquisition of Encysive,
Encysive’s change of control repurchase obligations under its $130 million, 2.5% convertible notes came into effect and, as such,
Encysive repurchased the convertible notes in consideration for their par value plus accrued interest in June 2008. In addition, in the
second quarter of 2008, we acquired Serenex, Inc. (Serenex), a privately held biotechnology company that owns SNX-5422, an oral
Heat Shock Protein 90 (Hsp90) inhibitor currently in phase I trials for the potential treatment of solid tumors and hematological
malignancies, and an extensive Hsp90 inhibitor compound library, which has potential uses in treating cancer and inflammatory and
neurodegenerative diseases. In connection with these acquisitions, we recorded approximately $170 million in Acquisition-related
in-process research and development charges and approximately $450 million in intangible assets.
•In the first quarter of 2008, we acquired CovX, a privately held biotherapeutics company specializing in preclinical oncology and
metabolic research and the developer of a biotherapeutics technology platform that we expect will enhance our biologic portfolio. Also
in the first quarter of 2008, we acquired all of the outstanding shares of Coley Pharmaceutical Group, Inc., (Coley), a biopharmaceutical
company specializing in vaccines and drug candidates designed to fight certain cancers, allergy and asthma disorders, and
autoimmune diseases, for approximately $230 million. In connection with these and two small acquisitions related to Animal Health, we
recorded approximately $440 million in Acquisition-related in-process research and development charges.
•In the first quarter of 2007, we acquired BioRexis Pharmaceutical Corp. (BioRexis), a privately held biopharmaceutical company with a
novel technology platform for developing new protein drug candidates, and Embrex, Inc. (Embrex), an animal health company that
possesses a unique vaccine delivery system known as Inovoject that improves consistency and reliability by inoculating chicks while
they are still in the egg. In connection with these and other smaller acquisitions, we recorded $283 million in Acquisition-related
in-process research and development charges.
•In February 2006, we completed the acquisition of the sanofi-aventis worldwide rights, including patent rights and production
technology, to manufacture and sell Exubera, an inhaled form of insulin, and the insulin-production business and facilities located in
Frankfurt, Germany, previously jointly owned by Pfizer and sanofi-aventis, for approximately $1.4 billion in cash (including transaction
costs). Substantially all assets recorded in connection with this acquisition have now been written off. See Note 4D. Certain Charges:
Exubera. Prior to the acquisition, in connection with our collaboration agreement with sanofi-aventis, we recorded a research and
development milestone due to us from sanofi-aventis of $118 million ($71 million, after tax) in 2006 in Research and development
expenses upon the approval of Exubera in January 2006 by the FDA.
•In December 2006, we completed the acquisition of PowderMed Ltd. (PowderMed), a U.K. company which specializes in the emerging
science of DNA-based vaccines for the treatment of influenza and chronic viral diseases, and in May 2006, we completed the
acquisition of Rinat Neurosciences Corp. (Rinat), a biologics company with several new central-nervous-system product candidates. In
2006, the aggregate cost of these and other smaller acquisitions was approximately $880 million (including transaction costs). In
connection with those transactions, we recorded $835 million in Acquisition-related in-process research and development charges.
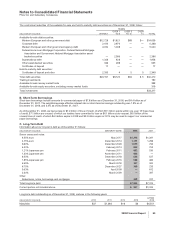
3. Discontinued Operations
We evaluate our businesses and product lines periodically for strategic fit within our operations.
In the fourth quarter of 2006, we sold our Consumer Healthcare business for $16.6 billion, and recorded a gain of approximately
$10.2 billion ($7.9 billion, net of tax) in Gains on sales of discontinued operations—net of tax in the consolidated statement of
income for 2006. In 2007, we recorded a loss of approximately $70 million, net of tax, primarily related to the resolution of
contingencies, such as purchase price adjustments and product warranty obligations, as well as pension settlements. This business
was composed of:
•substantially all of our former Consumer Healthcare segment;
•other associated amounts, such as purchase-accounting impacts, acquisition-related costs and restructuring and implementation costs
related to our cost-reduction initiatives that were previously reported in the Corporate/Other segment; and
2008 Financial Report 55