Pfizer 2006 Annual Report Download - page 70
Download and view the complete annual report
Please find page 70 of the 2006 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
2006 Financial Report 69
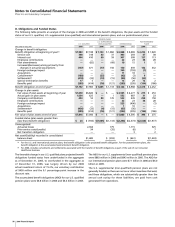
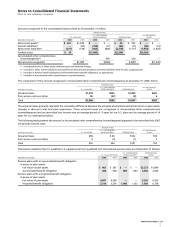
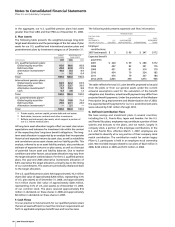
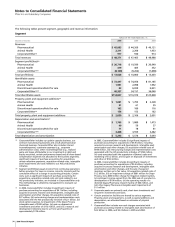
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
allegedly injured by Rezulin. This action was removed to the U.S.
District Court for the Eastern District of Louisiana in August 2005.
In 2005, both of the actions pending in the Eastern District of
Louisiana were transferred for consolidated pre-trial proceedings
to a Multi-District Litigation (In re Rezulin Products Liability Litigation
MDL-1348) in the U.S. District Court for the Southern District of New
York, where the action filed in April 2001 by Louisiana Health and
Eastern States Health had been brought.
A number of insurance carriers provided coverage for Rezulin
claims against Warner-Lambert. To date, we have entered into
settlements with several of those carriers for approximately $269
million. We have initiated and are pursuing arbitration
proceedings against the other carriers, who have denied coverage.
Asbestos
•
Quigley
Quigley Company, Inc. (Quigley), a wholly owned subsidiary, was
acquired by Pfizer in 1968 and sold small amounts of products
containing asbestos until the early 1970s. In September 2004, Pfizer
and Quigley took steps which, if approved by the courts and
claimants, will resolve all pending and future claims against Pfizer
and Quigley in which the claimants allege personal injury from
exposure to Quigley products containing asbestos, silica or mixed
dust. We took a charge of $369 million before-tax ($229 million after-
tax) to third quarter 2004 earnings in connection with these matters.
In September 2004, Quigley filed a petition in the U.S. Bankruptcy
Court for the Southern District of New York seeking reorganization
under Chapter 11 of the U.S. Bankruptcy Code. In March 2005,
Quigley filed a reorganization plan in the Bankruptcy Court that
must be approved by both the Bankruptcy Court and the U.S.
District Court for the Southern District of New York after receipt
of the vote of 75% of the claimants. In connection with that filing,
Pfizer entered into settlement agreements with lawyers
representing more than 80% of the individuals with claims related
to Quigley products against Quigley and Pfizer. The agreements
provide for a total of $430 million in payments, of which $215
million became due in December 2005 and is being paid to
claimants upon receipt by the Company of certain required
documentation from each of the claimants. The reorganization
plan, the approval of which is considered probable, will establish
a Trust for the payment of all remaining pending claims as well
as any future claims alleging injury from exposure to Quigley
products. Pfizer will contribute $405 million to the Trust through
a note, which has a present value of $172 million, as well as
approximately $100 million in insurance, and will forgive a $30
million secured loan to Quigley. If approved by the courts and the
claimants, the reorganization plan will result in a permanent
injunction directing all future claims alleging personal injury
from exposure to Quigley products to the Trust.
As certified by the balloting agent in May 2006, more than 75%
of Quigley’s claimants holding claims that represent more than
two-thirds in value of claims against Quigley voted to accept
Quigley’s plan of reorganization. On August 9, 2006, in reviewing
the voting tabulation methodology, the Bankruptcy Court ruled
that certain votes that accepted the plan were not predicated
upon the actual value of the claim. As a result, the reorganization
plan was not accepted. Quigley can adjust certain provisions in its
reorganization plan and the voting procedures to conform with
the Bankruptcy Court’s ruling, and then possibly re-solicit the
plan for acceptance or seek alternative remedies. These and
other options, including additional payments, are being
considered.
In a separately negotiated transaction with an insurance company
in August 2004, we agreed to a settlement related to certain
insurance coverage which provides for payments to us over a ten-
year period of amounts totaling $406 million.
•
Other Matters
Between 1967 and 1982, Warner-Lambert owned American Optical
Corporation, which manufactured and sold respiratory protective
devices and asbestos safety clothing. In connection with the sale of
American Optical in 1982, Warner-Lambert agreed to indemnify the
purchaser for certain liabilities, including certain asbestos-related and
other claims. As of December 31, 2006, approximately 110,200
claims naming American Optical and numerous other defendants
were pending in various federal and state courts seeking damages
for alleged personal injury from exposure to asbestos and other
allegedly hazardous materials. We are actively engaged in the
defense of, and will continue to explore various means to resolve,
these claims. Several of the insurance carriers that provided coverage
for the American Optical asbestos and other allegedly hazardous
materials claims have denied coverage. We believe that these
carriers’ position is without merit and are pursuing legal proceedings
against such carriers. Separately, there is a small number of lawsuits
pending against Pfizer in various federal and state courts seeking
damages for alleged personal injury from exposure to products
containing asbestos and other allegedly hazardous materials sold
by Gibsonburg Lime Products Company, which was acquired by
Pfizer in the 1960s and which sold small amounts of products
containing asbestos until the early 1970s. There also is a small
number of lawsuits pending in various federal and state courts
seeking damages for alleged exposure to asbestos in facilities
owned or formerly owned by Pfizer or its subsidiaries.
Hormone-Replacement Therapy
Pfizer and certain wholly owned subsidiaries and limited liability
companies, along with several other pharmaceutical manufacturers,
have been named as defendants in a number of lawsuits in various
federal and state courts alleging personal injury resulting from the
use of certain estrogen and progestin medications prescribed for
women to treat the symptoms of menopause. Plaintiffs in these suits
allege a variety of personal injuries, including breast cancer, stroke
and heart disease. Certain co-defendants in some of these actions
have asserted indemnification rights against Pfizer and its affiliated
companies. The cases against Pfizer and its affiliated companies
involve the products femhrt (which Pfizer divested in 2003), Activella
and Vagifem (which are Novo Nordisk products that were marketed
by a Pfizer affiliate from 2000 to 2004), and Provera, Ogen, Depo-
Estradiol, Estring and generic MPA, all of which remain approved
by the FDA for use in the treatment of menopause. The federal
court cases have been transferred for consolidated pre-trial
proceedings to a Multi-District Litigation (In re Prempro Products
Liability Litigation MDL-1507) in the U.S. District Court for the
Eastern District of Arkansas.