Pfizer 2006 Annual Report Download - page 23
Download and view the complete annual report
Please find page 23 of the 2006 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
2006 Financial Report 21
Financial Review
Pfizer Inc and Subsidiary Companies
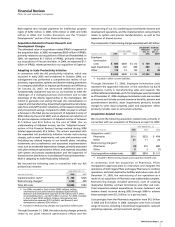
Ongoing or planned clinical trials for additional uses
and dosage forms for our products include:
PRODUCT INDICATION
Geodon/
Zeldox Bipolar relapse prevention; bipolar pediatric
Lyrica Generalized anxiety disorder; epilepsy
monotherapy
Revatio Pediatric pulmonary arterial hypertension
Macugen Diabetic macular edema
Drug candidates in late-stage development include CP-945,598 a
cannabinoid-1 receptor antagonist for treatment of obesity;
axitinib, a multi-targeted receptor kinase for treatment of thyroid
cancer; Zithromax/chloroquine for treatment of malaria; PF-
3,512,676, a toll-like receptor 9 agonist for non-small cell lung
cancer developed in partnership with Coley; CP-675,206, an anti-
CTLA4 monoclonal antibody for melanoma; and Sutent for
treatment of metastatic breast cancer.
On December 2, 2006, we announced that in the interests of
public safety, we were stopping all torcetrapib clinical trials and
had informed the FDA. Based on the recommendation of the
independent Data Safety Monitoring Board, we have terminated
the ILLUMINATE morbidity and mortality study for torcetrapib due
to an imbalance of mortality and cardiovascular events and asked
all clinical investigators to inform patient participants to stop
taking the study medication immediately. In addition, we have
ended the development program for this compound.
On November 28, 2006, we announced that we and Akzo Nobel’s
Organon healthcare unit agreed to discontinue our collaboration
in the further development of asenapine, a drug candidate for the
treatment for schizophrenia and bipolar disorder. Our decision to
discontinue participation in the asenapine development program
was an outcome of a commercial analysis of the compound as part
of our overall portfolio. We will return all product rights,
intellectual property and data to Organon in 2007.
Additional product-related programs are in various stages of
discovery and development. Also, see our discussion in the “Our
Strategic Initiatives—Strategy and Recent Transactions: Acquisitions,
Licensing and Collaborations” section of this Financial Review.
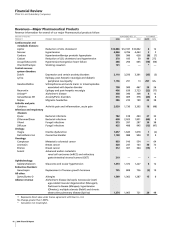
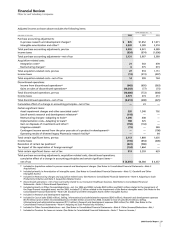
Animal Health
Revenues of our Animal Health business follow:
YEAR ENDED DEC. 31, % CHANGE
__________________________________________ _________________
(MILLIONS OF DOLLARS) 2006 2005 2004 06/05 05/04
Livestock products $1,458 $1,379 $1,200 6 15
Companion animal
products 853 827 753 3 10
Total Animal Health $2,311 $2,206 $1,953 5 13
Our Animal Health business is one of the largest in the world.
The increase in Animal Health revenues in 2006, as compared to
2005, was primarily attributable to:
•
for livestock products, the continued good performance of
Draxxin (for treatment of respiratory disease in cattle and
swine) in Europe and in the U.S.; and
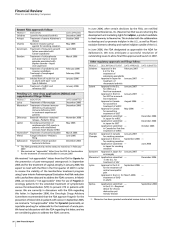
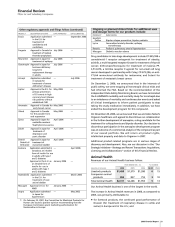
Other regulatory approvals and filings follow: (continued)
PRODUCT DESCRIPTION OF EVENT DATE APPROVED DATE SUBMITTED
Eraxis Application submitted — September 2006
in the E.U. for
treatment of
candidemia and
candidiasis
Fragmin
Approval in Canada for
July 2006 —
treatment of medical
thrombo-prophylaxis
Neurontin Approval in Japan for July 2006 —
treatment of epilepsy
Genotropin Approval in Japan for July 2006 —
hormone deficiency
long-term
replacement therapy
in adults
Aricept Application submitted — July 2006
in Canada for
treatment of severe
Alzheimer’s disease
Lipitor Approval in the E.U. for May 2006 —
primary prevention
of CV events in high
coronary heart disease
risk patients without
established CHD
Aromasin Approval in Canada for May 2006 —
early breast cancer
Vfend Approval in Canada May 2006 —
for the powder form
oral suspension
Zyvox Approval in Japan for April 2006 —
methicillin-resistant
Staphylococcus aureus
Zoloft Approval in Japan for April 2006 —
treatment of
depression and
panic disorder
Detrol/ Approval in Japan for April 2006 —
Detrol LA/ treatment of
Detrusitol overactive bladder
Exubera Application submitted in — April 2006
Canada as an inhaled
form of insulin for use
in adults with type 1
and 2 diabetes
Approval in the E.U. as January 2006 —
an inhaled form of
insulin for use in
adults with type 1
and 2 diabetes
Fesoterodine (b)
Application submitted — March 2006
in the E.U. for
treatment of over-
active bladder
Macugen Approval in E.U. for January 2006 —
AMD
Inspra Application submitted — May 2002
in Japan for
hypertension
(b)
On February 23, 2007, the Committee for Medicinal Products for
Human Use issued a positive opinion recommending that the
European Commission grant marketing authorization for
fesoterodine in Europe.