Pfizer 2006 Annual Report Download - page 22
Download and view the complete annual report
Please find page 22 of the 2006 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
20 2006 Financial Report
Financial Review
Pfizer Inc and Subsidiary Companies
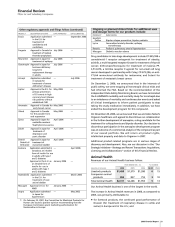
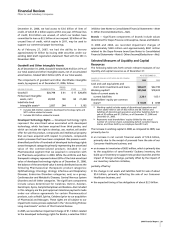
Recent FDA approvals follow:
PRODUCT INDICATION DATE APPROVED
Celebrex Juvenile rheumatoid arthritis December 2006
Aricept Treatment of severe Alzheimer’s October 2006
disease
Chantix Nicotine-receptor partial May 2006
agonist for smoking cessation
Genotropin
Treatment of long-term growth April 2006
failure associated with
Turner’s syndrome
Geodon Treatment of schizophrenia March 2006
and acute manic or mixed
episodes associated with
bipolar disorder—liquid
oral suspension
Eraxis Treatment of candidemia and February 2006
invasive candidiasis
Treatment of esophageal February 2006
candidiasis
Exubera Inhaled form of insulin for use January 2006
in adults with type 1 and
type 2 diabetes
Sutent Treatment of mRCC and January 2006
refractory GIST
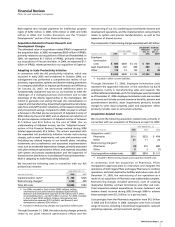
Pending U.S. new drug applications (NDAs) and
supplemental filings follow:
PRODUCT INDICATION DATE SUBMITTED
Lyrica Treatment of fibromyalgia December 2006
Maraviroc
(a)
Treatment of human immuno- December 2006
deficiency virus/acquired
immune deficiency (HIV)
in treatment-experienced
patients
Zithromax
Bacterial infections—sustained
November 2006
release—Pediatric filing
Lipitor Secondary prevention of May 2006
cardiovascular (CV) events in
patients with established
coronary heart disease (CHD)
Fesoterodine
(b)
Treatment of overactive bladder March 2006
Vfend Fungal infections—Pediatric June 2005
filing
dalbavancin
Treatment of Gram-positive December 2004
bacterial infections
(a)
The FDA granted priority review status to maraviroc in February
2007.
(b)
We received an “approvable” letter from the FDA for fesoterodine
for the treatment of overactive bladder in January 2007.
We received “not-approvable” letters from the FDA for Oporia for
the prevention of post-menopausal osteoporosis in September
2005 and for the treatment of vaginal atrophy in January 2006. We
expect to meet with the FDA in the first quarter of 2007 in order
to review the viability of the lasofoxifene treatment program
using 3-year interim Postmenopausal Evaluation And Risk-reduction
with Lasofoxifene data and to address the FDA’s concerns. In March
2006, we received a “not-approvable” letter for use of Fragmin in
oncology patients for the extended treatment of symptomatic
venous thromboembolism (VTE) to prevent VTE in patients with
cancer. We are currently in discussions with the FDA regarding
this letter. In September 2006, the Oncologic Drugs Advisory
Committee recommended that the FDA approve Fragmin for the
prevention of blood clots in patients with cancer. In September 2005,
we received a “not-approvable” letter for Dynastat (parecoxib), an
injectable prodrug for valdecoxib for the treatment of acute pain.
We have had discussions with the FDA regarding this letter, and we
are considering plans to address the FDA’s concerns.
(a)
Maraviroc has been granted accelerated review status in the E.U.
In June 2006, after certain decisions by the FDA, we notified
Neurocrine Biosciences, Inc. (Neurocrine) that we are returning the
development and marketing rights for indiplon, a product candidate
to treat insomnia, to Neurocrine. This includes both the collaboration
to develop and co-promote indiplon in the U.S., as well as Pfizer’s
exclusive license to develop and market indiplon outside of the U.S.
In June 2006, the FDA designated as approvable the NDA for
dalbavancin. We now anticipate a successful resolution of
outstanding issues to allow final FDA approval and launch in 2007.
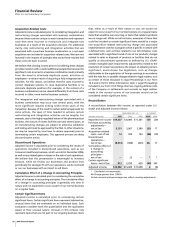
Other regulatory approvals and filings follow:
PRODUCT DESCRIPTION OF EVENT DATE APPROVED DATE SUBMITTED
Celebrex Approval in the February 2007 —
E.U. for the
treatment of
ankylosing spondylitis
Approval in Japan for January 2007 —
treatment of
rheumatoid arthritis
Sutent Approval in the E.U. January 2007 —
for mRCC as a
first-line treatment
Approval in the E.U. January 2007 —
for GIST as a second-
line treatment
Approval in Canada August 2006 —
for second-line
treatment of mRCC
Approval in Canada May 2006 —
for second-line
treatment of GIST
Application submitted — December 2006
in Japan for mRCC
Application submitted — December 2006
in Japan for GIST
Application submitted — October 2006
in Canada for first-line
treatment of mRCC
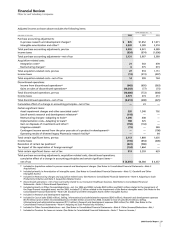
Chantix/ Approval in Canada January 2007 —
Champix for smoking cessation
Approval in the E.U. September 2006 —
for smoking cessation
Application submitted — June 2006
in Japan for smoking
cessation
Somavert Approval in Japan for January 2007 —
acromegaly
Maraviroc
(a)
Application submitted — December 2006
in the E.U. for
treatment of HIV
Lyrica
Approval in the E.U.
September 2006 —
for the treatment of
central neuropathic
pain
Approval in the E.U. for March 2006 —
treatment of GAD
in adults
Spiriva Application submitted — September 2006
in the E.U.—Respimat
device for chronic
obstructive pul-
monary disease