Pfizer 2006 Annual Report Download - page 30
Download and view the complete annual report
Please find page 30 of the 2006 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
28 2006 Financial Report
Financial Review
Pfizer Inc and Subsidiary Companies
Financial Condition, Liquidity and
Capital Resources
Net Financial Assets
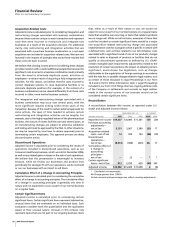
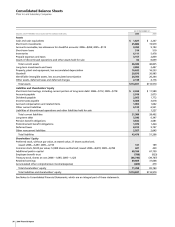
Our net financial asset position as of December 31 follows:
(MILLIONS OF DOLLARS) 2006 2005
Financial assets:
Cash and cash equivalents $ 1,827 $ 2,247
Short-term investments 25,886 19,979
Short-term loans 514 510
Long-term investments and loans 3,892 2,497
Total financial assets 32,119 25,233
Debt:
Short-term borrowings, including
current portion of long-term debt 2,434 11,589
Long-term debt 5,546 6,347
Total debt 7,980 17,936
Net financial assets $24,139 $ 7,297
The increase in net financial assets reflects the proceeds from the
sale of our Consumer Healthcare business for $16.6 billion. The
change in the composition of our net financial assets also reflects
the use of redemptions of short-term investments to pay down
short-term borrowings.
We rely largely on operating cash flow, long-term debt and short-
term commercial paper borrowings to provide for the working
capital needs of our operations, including our R&D activities. We
believe that we have the ability to obtain both short-term and long-
term debt to meet our financing needs for the foreseeable future.
Impact of Repatriation of Foreign Earnings
In 2005, under the Jobs Act, we repatriated to the U.S. approximately
$37 billion in cash from foreign earnings (see the “Provision/(Benefit)
for Taxes on Income” section of this Financial Review). This cash is
being used for domestic expenditures relating to advertising and
marketing activities, research and development activities, capital
assets and other asset acquisitions and non-executive compensation
in accordance with the provisions of the Jobs Act. The repatriation
resulted in a decrease in short-term and long-term investments
held overseas as the cash was repatriated and an increase in short-
term borrowings overseas was used to fund the repatriation.
Investments
Our short-term and long-term investments consist primarily of
mutual funds invested in debt financial instruments and high
quality, liquid investment-grade available-for-sale debt securities.
Our long-term investments include debt securities that totaled $2.1
billion as of December 31, 2006, which have maturities ranging
substantially from one to ten years. Wherever possible, cash
management is centralized and intercompany financing is used
to provide working capital to our operations. Where local
restrictions prevent intercompany financing, working capital
needs are met through operating cash flows and/or external
borrowings. Our portfolio of short-term investments was reduced
in the first quarter of 2006 by about $7 billion and the proceeds
were primarily used to pay down short-term borrowings. In late
December 2006, our portfolio of short-term investments increased
by $16.6 billion, reflecting the receipt of proceeds from the sale
of our Consumer Healthcare business.
Long-Term Debt Issuance
On February 22, 2006, we issued the following Japanese yen
fixed-rate bonds, to be used for general corporate purposes:
•
$508 million equivalent, senior unsecured notes, due February
2011, which pay interest semi-annually, beginning on August
22, 2006, at a rate of 1.2%; and
•
$466 million equivalent, senior unsecured notes, due February
2016, which pay interest semi-annually, beginning on August
22, 2006, at a rate of 1.8%.
The notes were issued under a $5 billion debt shelf registration
filed with the SEC in November 2002.
Long-Term Debt Redemption
In May 2006, we decided to exercise our option to call, at par-value
plus accrued interest, $1 billion of senior unsecured floating-rate
notes, which were included in Long-term debt as of December 31,
2005. Notice to call was given to the Trustees and the notes were
redeemed in the third quarter of 2006.
Credit Ratings
Two major corporate debt-rating organizations, Moody’s Investors
Services (Moody’s) and Standard & Poor’s (S&P), assign ratings to
our short-term and long-term debt. The following chart reflects
the current ratings assigned to our senior unsecured non-credit
enhanced long-term debt and commercial paper issued directly
by us by each of these agencies:
NAME OF COMMERCIAL
LONG-TERM DEBT
DATE OF LAST______________________
RATING AGENCY PAPER RATING OUTLOOK ACTION
Moody’s P-1 Aa1 Stable December 2006
S&P A1+ AAA Negative December 2006
On December 19, 2006, Moody’s downgraded our long-term debt
rating to Aa1, its second highest investment grade rating, following
a review initiated on December 4, 2006, citing our announcement
on December 2, 2006, that we were ceasing development of
torcetrapib. The downgrade reflects Moody’s assessment that the
relationship between our patent exposures and our pipeline
strength is no longer consistent with a Moody’s Aaa rating.
Following our December 2, 2006 announcement of our cessation
of development of torcetrapib, S&P changed our rating outlook
from stable to negative, noting a slowdown in sales and earnings
growth as a result of major patent expirations and increased
competition. S&P continues to rate our long-term debt at AAA, its
highest investment grade rating, relying on our excellent position
in the worldwide pharmaceutical market, highlighted by our
diverse drug portfolio and large scale R&D program, together
with our superior financial profile and cash-generating ability.
Our access to financing at favorable rates would be affected by
a substantial downgrade in our credit ratings.
Debt Capacity
We have available lines of credit and revolving-credit agreements
with a group of banks and other financial intermediaries. We
maintain cash balances and short-term investments in excess of
our commercial paper and other short-term borrowings. As of