Pfizer 2006 Annual Report Download - page 20
Download and view the complete annual report
Please find page 20 of the 2006 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
18 2006 Financial Report
Financial Review
Pfizer Inc and Subsidiary Companies
The U.S. Patent and Trademark Office granted a five-year
extension to the Geodon U.S. patent, extending its exclusivity
to 2012.
•
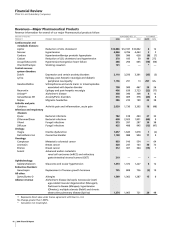
Lyrica achieved $1.2 billion in worldwide revenues in 2006,
continuing its performance as one of Pfizer’s most successful
pharmaceutical launches. In September 2006, Lyrica was
approved by the European Commission to treat central nerve
pain, which is associated with conditions such as spinal injury,
stroke and multiple sclerosis. In addition, in March 2006, it
was approved by the European Commission to treat generalized
anxiety disorder (GAD) in adults, thereby providing a new
treatment option for the approximately 12 million Europeans
living with GAD.
Lyrica was approved by the FDA in June 2005 for adjunctive
therapy for adults with partial onset epileptic seizures. This
indication built on the earlier FDA approval of Lyrica for two
of the most common forms of neuropathic pain; painful diabetic
peripheral neuropathy, a chronic neurologic condition affecting
about three million Americans, and post-herpetic neuralgia.
Lyrica was launched in the U.S., Canada and Italy in September
2005 and is now approved in 77 countries and available in 59
markets. As of December 2006, more than four million patients
have been prescribed Lyrica since its introduction. Lyrica gained
a 9.6% new prescription share of the total U.S. anti-epileptic
market in December 2006.
•
Celebrex achieved an 18% increase in worldwide sales in 2006
compared to 2005. In the U.S., Celebrex had a monthly new
prescription share of 11.1% in December 2006. Pfizer is
continuing its efforts to address physicians’ and patients’
questions by clearly communicating the risks and benefits of
Celebrex. In addition, the Prospective Randomized Evaluation
of Celecoxib Integrated Safety vs. Ibuprofen or Naproxen
(PRECISION) study, which began enrolling patients in October
2006, will provide further understanding of the comparative
cardiovascular safety of Celebrex and some common non-
specific non-steroidal anti-inflammatory drugs (NSAIDs) in
arthritis patients at risk for, or already suffering from, heart
disease.
Pfizer began to reintroduce branded advertising in the U.S. in
April 2006 in alignment with our new direct-to-consumer (DTC)
advertising principles, highlighting Celebrex’s strong clinical
profile and benefits. In August 2006, Celebrex was granted
pediatric exclusivity in the U.S., extending its patent protection
until May 2014. Celebrex was approved by the FDA for juvenile
rheumatoid arthritis in December 2006. In January 2007,
Celebrex was approved in Japan for the treatment of
osteoarthritis and rheumatoid arthritis. In February 2007,
Celebrex was approved in Europe for the treatment of
ankylosing spondylitis.
In 2005, in accordance with decisions by applicable regulatory
authorities, we implemented label changes for Celebrex in
the U.S. and the E.U. The revised U.S. label for Celebrex contains
a boxed warning of potential serious cardiovascular and
gastrointestinal risks that is consistent with warnings for all
other prescription NSAIDS. The revised E.U. labels for Celebrex
and all other COX-2 medicines include a restriction on use by
patients with established heart disease or stroke and additional
warnings to physicians regarding use by patients with
cardiovascular risk factors.
See Notes to Consolidated Financial Statements—Note 19.
Legal Proceedings and Contingencies for a discussion of recent
developments with respect to certain patent litigation relating
to Celebrex.
•
Zithromax experienced a 69% decline in worldwide sales in 2006
compared to 2005, reflecting the expiration of its composition-
of-matter patent in the U.S. in November 2005 and the end of
Pfizer’s active sales promotion in July 2005. During the fourth
quarter of 2005, four generic versions of oral solid azithromycin
were launched, including an authorized generic by Pfizer’s
Greenstone subsidiary. Additional generic formulations of
azithromycin were launched during 2006, including three oral
suspensions and two intravenous versions, and a third
intravenous version is expected to be launched in 2007.
•
Eraxis, an antifungal approved to treat candidemia and other
forms of Candida infections (intra-abdominal abscesses and
peritonitis), as well as esophageal candidiasis, was launched mid-
June 2006 in the U.S. Candidemia is the most deadly of the
common hospital-acquired bloodstream infections with a
mortality rate of approximately 40%.
•
Viagra remains the leading treatment for erectile dysfunction
and one of the world’s most recognized pharmaceutical brands,
with more than 58% of U.S. total prescriptions in the erectile
dysfunction market through December 2006. Viagra sales grew
1% worldwide in 2006 compared to 2005. We expect to see
continued pressure on sales in the U.S. More than 45 states have
either eliminated erectile-dysfunction coverage or have enacted
“preferred drug lists” that have the potential to limit Pfizer sales
to state Medicaid programs. Effective January 1, 2006, federal
funds may not be used for reimbursement of erectile-
dysfunction medications by the Medicaid program. Medicare
coverage of Viagra will end in 2007.
Pfizer has introduced new branded and unbranded advertising
to encourage men with erectile dysfunction to talk to their
physicians about their condition.
•
Detrol/Detrol LA, a muscarinic receptor antagonist, is the most
prescribed medicine for overactive bladder, a condition that
affects up to 100 million people around the world. Detrol/Detrol
LA is an extended-release formulation taken once daily.
Worldwide Detrol/Detrol LA sales grew 11% to $1.1 billion in
2006. Detrol/Detrol LA continues to lead the overactive bladder
market and perform well in an increasingly competitive
marketplace. In the U.S., Detrol/Detrol LA’s new prescription
share grew 2% to a 43.2% share for the full year 2006. A
strong clinical database, unparalleled access in managed care
and Medicare, and a history of delivering positive patient
outcomes have enabled Detrol/Detrol LA to maintain market
share, and remain the clear first-line antimuscarinic agent
among both primary care physicians and urologists. See Notes
to Consolidated Financial Statements—Note 19. Legal
Proceedings and Contingencies for a discussion of recent