Amgen 2012 Annual Report Download - page 21
Download and view the complete annual report
Please find page 21 of the 2012 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
14
is a protein found in all human cells. Some colorectal cancers have mutations in the KRAS gene. Vectibix® has been shown to be
ineffective in people whose tumors had KRAS mutations in codon 12 or 13.
In 2009, we announced results from the ‘203 and ‘181 pivotal phase 3 trials evaluating Vectibix® in combination with
chemotherapy (FOLFOX or FOLFIRI) as a first- and second-line treatment for mCRC, respectively. Both studies demonstrated
that Vectibix® administered with chemotherapy significantly improved progression-free survival in patients with wild-type KRAS
mCRC. Additionally, both studies showed numeric improvements in median overall survival in the same patient population. The
numeric improvements in median overall survival failed to achieve statistical significance. It was previously agreed with the FDA
that the '181 study would serve as the confirmatory trial for establishing full approval for the mCRC indication.
In July 2011, we announced that we received Complete Response Letters from the FDA on the first- and second-line mCRC
sBLAs that we filed in late 2010. The FDA did not ask for new clinical studies but did request an updated safety analysis and
additional analyses of the overall survival data in the ’ 181 and ’ 203 studies using more mature data sets. The FDA has also informed
us that approval for the first- and second-line mCRC indications will be contingent upon approval of the companion diagnostic
device being developed in collaboration with QIAGEN N.V. (QIAGEN), which identifies a patient’ s KRAS gene status. We are
currently working on addressing the FDA’ s requests in the Complete Response Letters.
In November 2011, the EC approved a variation to the marketing authorization for Vectibix® to include indications for the
treatment of patients with wild-type KRAS mCRC in first- and second-line in combination with chemotherapy.
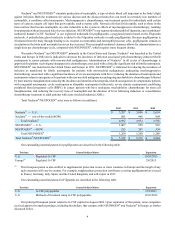
Total Vectibix® sales were as follows (in millions):
2012 2011 2010
Total Vectibix®$ 359 $ 322 $ 288
Our outstanding material patents for panitumumab are described in the following table.
Territory General Subject Matter Expiration
U.S. Human monoclonal antibodies to EGFr 4/8/2020
U.S. Human monoclonal antibodies to EGFr 5/5/2017
Europe Fully human antibodies that bind EGFr 12/3/2017
Europe(1) Human monoclonal antibodies to EGFr 5/5/2018
(1) This European patent is also entitled to supplemental protection in one or more countries in Europe and the length of any
such extension will vary by country. For example, supplementary protection certificates covering panitumumab have issued
in France, Italy, Spain, and the United Kingdom, and will expire in 2022.
Any products or technologies that are directly or indirectly successful in treating mCRC after disease progression either on
or following fluoropyrimidine-, oxaliplatin- and irinotecan-containing chemotherapy regimens could negatively impact Vectibix®
sales.
The following table reflects companies and their currently marketed products that compete with Vectibix® in the United
States and Europe and may not be exhaustive.
Territory Competitor Marketed Product Competitor
U.S. Erbitux®Eli Lilly/BMS
U.S. Zaltrap®Sanofi
U.S. Avastin®Genentech, Inc. (Genentech)
U.S. Stivarga®Bayer
Europe Erbitux®Merck KGaA
Nplate® (romiplostim)
In August 2008, the FDA approved Nplate® for the treatment of thrombocytopenia in splenectomized (spleen removed) and
non-splenectomized adults with chronic immune thrombocytopenic purpura (ITP). Nplate® works by raising and sustaining platelet
counts. We were granted an exclusive license by K-A to manufacture and market Nplate® in the United States, all European
countries, Canada, Australia, New Zealand, Mexico, all Central and South American countries and certain countries in Central
Asia, Africa and the Middle East. In February 2009, we announced that the EC had granted marketing authorization for Nplate®