Amgen 2012 Annual Report Download - page 20
Download and view the complete annual report
Please find page 20 of the 2012 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
13
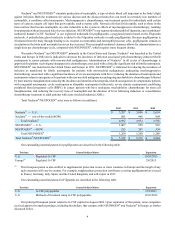
Our outstanding material patents for cinacalcet are described in the following table.
Territory General Subject Matter Expiration
U.S. Calcium receptor-active molecules including species 10/23/2015
U.S. Calcium receptor-active molecules 3/8/2018
U.S. Methods of treatment 12/14/2016
Europe(1) Calcium receptor-active molecules 10/23/2015
(1) This European patent is also entitled to supplemental protection in one or more countries in Europe and the length of any
such extension will vary by country. For example, supplementary protection certificates covering cinacalcet have issued in
France, Germany, Italy, Spain, and the United Kingdom, and will expire in 2019.
Any products or technologies that are directly or indirectly successful in treating secondary hyperparathyroidism in patients
with CKD on dialysis and/or hypercalcemia in patients with parathyroid carcinoma could negatively impact Sensipar®/Mimpara®
sales.
The following table reflects companies and their currently marketed products that compete with Sensipar® in the United
States and with Mimpara® in Europe in the nephrology segment for patients with CKD on dialysis and may not be exhaustive.
Territory Competitor Marketed Product Competitor
U.S. Hectorol®Genzyme Corporation (Genzyme)
U.S. Rocaltrol®Roche
U.S. Calcijex®Abbott (1)
U.S. Calcium Acetate®Roxane Laboratories/Sandoz
U.S. & Europe Zemplar®Abbott (1)
U.S. & Europe Renagel®Genzyme
U.S. & Europe Renvela®Genzyme
U.S. & Europe PhosLo®/Rephoren®Fresenius Medical Care AG & Co. KGaA (Fresenius Medical Care)
U.S. & Europe OsvaRen®Fresenius Medical Care
U.S. & Europe Fosrenol®Shire Pharmaceuticals Group Plc
(1) In January 2013, Abbott announced that it completed the separation of its research-based pharmaceuticals business, which
became AbbVie, a new independent biopharmaceutical company which now owns the rights to this product.
In July 2008, we filed a lawsuit against Teva and Barr Pharmaceuticals Inc. (Barr) for infringement of four Sensipar® patents.
The lawsuit was based on Abbreviated New Drug Applications (NDA) filed by Teva and Barr that sought approval to market
generic versions of Sensipar®. Following trial, in January 2011, the U.S. District Court for the District of Delaware granted an
injunction prohibiting Teva and Barr from commercializing generic versions of Sensipar® in the United States until expiration of
three of those patents. These generic versions could compete with Sensipar® in the future.
Vectibix® (panitumumab)
Vectibix® is our registered trademark for panitumumab, our monoclonal antibody for the treatment of patients with EGFr
expressing metastatic colorectal cancer (mCRC) after disease progression on, or following fluoropyrimidine-, oxaliplatin- and
irinotecan- containing chemotherapy regimens. EGFr is a protein that plays an important role in cancer cell signaling and is over-
expressed in many human cancers. Vectibix® binds with high affinity to EGFrs and interferes with signals that might otherwise
stimulate growth and survival of the cancer cell. In September 2006, Vectibix® received FDA accelerated approval in the United
States, based upon clinical trial data from a study demonstrating a statistically significant improvement in progression-free survival
and with the condition that Amgen conduct a confirmatory trial to verify the clinical benefit of panitumumab through demonstration
of an improvement in overall survival. (See discussion of the '181 trial below.) In the EU, the conditional approval of Vectibix®
as monotherapy, for the treatment of patients with EGFr expressing metastatic colorectal carcinoma with non-mutated (wild-type)
KRAS genes after failure of fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy regimens, was received in
December 2007 and is reviewed annually by the Committee for Medicinal Products for Human Use (CHMP). Each year thereafter,
the EU conditional marketing authorization was renewed with an additional specific obligation to conduct a clinical trial in the
approved monotherapy indication. In 2010, we began enrollment for this additional clinical trial which compares the effect of
Vectibix® versus Erbitux® (cetuximab) on overall survival for chemorefractory mCRC patients with wild-type KRAS genes. KRAS