Amgen 2012 Annual Report Download - page 11
Download and view the complete annual report
Please find page 11 of the 2012 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
4
Neulasta® and NEUPOGEN® stimulate production of neutrophils, a type of white blood cell important in the body’ s fight
against infection. Both the treatments for various diseases and the diseases themselves can result in extremely low numbers of
neutrophils, a condition called neutropenia. Myelosuppressive chemotherapy, one treatment option for individuals with certain
types of cancers, targets cell types that grow rapidly, such as tumor cells. Normal cells that divide rapidly, such as those in the
bone marrow that become neutrophils, are also vulnerable to the cytotoxic effects of myelosuppressive chemotherapy, resulting
in neutropenia with an increased risk of severe infection. NEUPOGEN® is our registered trademark for Filgrastim, our recombinant-
methionyl human G-CSF. Neulasta® is our registered trademark for pegfilgrastim, a pegylated protein based on the Filgrastim
molecule. A polyethylene glycol molecule is added to the Filgrastim molecule to make pegfilgrastim. Because pegfilgrastim is
eliminated from the body through binding to its receptor on neutrophils and neutrophil precursor cells, pegfilgrastim remains in
circulation in the body until neutrophil recovery has occurred. This neutrophil-mediated clearance allows for administration as a
single dose per chemotherapy cycle, compared with NEUPOGEN®, which requires more frequent dosing.
We market Neulasta® and NEUPOGEN® primarily in the United States and Europe. Neulasta® was launched in the United
States and Europe in 2002 and is indicated to decrease the incidence of infection associated with chemotherapy-induced febrile
neutropenia in cancer patients with non-myeloid malignancies. Administration of Neulasta® in all cycles of chemotherapy is
approved for patients receiving myelosuppressive chemotherapy associated with a clinically significant risk of febrile neutropenia.
NEUPOGEN® was launched in the United States and Europe in 1991. NEUPOGEN® is indicated for reducing the incidence of
infection as manifested by febrile neutropenia for patients with non-myeloid malignancies undergoing myelosuppressive
chemotherapy associated with a significant incidence of severe neutropenia with fever; reducing the duration of neutropenia and
neutropenia-related consequences for patients with non-myeloid malignancies undergoing myeloablative chemotherapy followed
by bone marrow transplantation; reducing the incidence and duration of neutropenia-related consequences in symptomatic patients
with congenital neutropenia, cyclic neutropenia or idiopathic neutropenia (collectively, severe chronic neutropenia); mobilizing
peripheral blood progenitor cells (PBPC) in cancer patients who have undergone myeloablative chemotherapy for stem cell
transplantation; and reducing the recovery time of neutrophils and the duration of fever following induction or consolidation
chemotherapy treatment in adult patients with acute myeloid leukemia (AML).
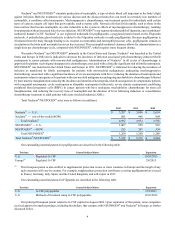
Total Neulasta®/NEUPOGEN® sales were as follows (in millions):
2012 2011 2010
Neulasta® — U.S. $ 3,207 $ 3,006 $ 2,654
Neulasta® — rest-of-the-world (ROW) 885 946 904
Total Neulasta®4,092 3,952 3,558
NEUPOGEN® — U.S. 1,007 959 932
NEUPOGEN® — ROW 253 301 354
Total NEUPOGEN®1,260 1,260 1,286
Total Neulasta®/NEUPOGEN®$ 5,352 $ 5,212 $ 4,844
Our outstanding material patents for pegfilgrastim are described in the following table.
Territory General Subject Matter Expiration
U.S. Pegylated G-CSF 10/20/2015
Europe(1) Pegylated G-CSF 2/8/2015
(1) This European patent is also entitled to supplemental protection in one or more countries in Europe and the length of any
such extension will vary by country. For example, supplementary protection certificates covering pegfilgrastim have issued
in France, Germany, Italy, Spain, and the United Kingdom, and will expire in 2017.
Our outstanding material patents for Filgrastim are described in the following table.
Territory General Subject Matter Expiration
U.S. G-CSF polypeptides 12/3/2013
U.S. Methods of treatment using G-CSF polypeptides 12/10/2013
Our principal European patent related to G-CSF expired in August 2006. Upon expiration of that patent, some companies
received approval to market products, including biosimilars, that compete with NEUPOGEN® and Neulasta® in Europe, as further
discussed below.