Amgen 2012 Annual Report Download - page 18
Download and view the complete annual report
Please find page 18 of the 2012 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
11
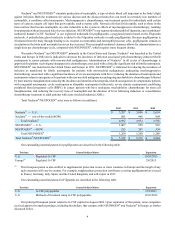
The following table reflects currently marketed products that compete with XGEVA®. The table below and the following
discussion of competitor products in development may not be exhaustive.
Territory Competitor Marketed Product Competitor
U.S. & Europe Zometa®(1) Novartis AG (Novartis)
U.S. & Europe Aredia®(2) Novartis
(1) Novartis has indicated that patent protection on the active ingredient for Zometa® will expire in 2013 in the United States.
At such time, we expect that generic forms of zoledronic acid may become commercially available and compete with Zometa®
and XGEVA®. Generic forms of zoledronic acid became available in other major markets in 2012.
(2) This product has lost its patent protection and generic versions of this product are available.
In addition, Bayer has filed with the FDA for approval of alpharadin for the treatment of castration-resistant prostate cancer
patients with bone metastases, that may compete with XGEVA® in the future.
Prolia®
In June 2010, the FDA approved Prolia® for the treatment of postmenopausal women with osteoporosis at high risk for
fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture, or patients who have failed or are
intolerant to other available osteoporosis therapy. In September 2011, we announced that the FDA approved two additional
indications for Prolia® as a treatment to increase bone mass in women at high risk for fracture receiving adjuvant aromatase
inhibitor therapy for breast cancer and as a treatment to increase bone mass in men at high risk for fracture receiving androgen
deprivation therapy for non-metastatic prostate cancer. In September 2012, the FDA approved Prolia® for a treatment to increase
bone mass in men with osteoporosis at high risk for fracture.
In May 2010, the EC granted marketing authorization for Prolia® for the treatment of osteoporosis in postmenopausal women
at increased risk of fractures and for the treatment of bone loss associated with hormone ablation in men with prostate cancer at
increased risk of fractures.
Any products or technologies that are directly or indirectly successful in treating osteoporosis in patients at high risk for
fracture could negatively impact Prolia® sales.
The following table and discussion reflect other companies and their currently marketed products that compete with Prolia®.
The table below and the following discussion of competitor marketed products and products in development may not be exhaustive.
Territory Competitor Marketed Product Competitor
U.S. & Europe FOSAMAX®(1) Merck
U.S. & Europe Actonel®/Atelvia™Warner Chilcott PLC
U.S. & Europe Boniva®(1)/Bonviva®(1) Roche
U.S. & Europe Evista®Eli Lilly
U.S. & Europe Forteo®/Forsteo™Eli Lilly
U.S. & Europe Miacalcin®Novartis
U.S. & Europe Aclasta®(1)/Reclast®Novartis
Europe Conbriza®Pfizer
Europe Fablyn®Pfizer
(1) This product has lost its patent protection and generic versions of this product are available.
We expect several additional marketed products noted above to lose patent protection over the next several years.
Merck (odanacatib) and Radius Health, Inc. (BA058) have product candidates in phase 3 clinical development for PMO.