Amgen 2012 Annual Report Download - page 15
Download and view the complete annual report
Please find page 15 of the 2012 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
8
Certain of these developments have had a material adverse impact on sales of our ESAs.
In addition, in November 2011, we entered into a seven-year supply agreement with DaVita Inc. (DaVita), commencing
January 1, 2012, to supply EPOGEN® in amounts necessary to meet no less than 90% of DaVita’s and its affiliates’ requirements
for ESAs used in providing dialysis services in the United States and Puerto Rico.
We have an ongoing oncology pharmacovigilance program in place for Aranesp®. The five clinical trials originally included
in the program explored the use of ESAs in settings different from those outlined in the FDA approved label and were designated
by the FDA as PMCs. Of the five studies, one was sponsored by Amgen while the other four were investigator-sponsored. Four
of the studies are complete and analysis of the results from the fifth study is currently ongoing. The results of certain of those
studies contributed to safety-related product labeling changes for our ESAs and changes in reimbursement, as noted above. In
addition, Janssen Research & Development, LLC (JRD), a subsidiary of J&J, and/or its investigators have conducted numerous
studies that contribute to the understanding of ESA safety. Results of the JRD studies were submitted to the FDA.
Additionally, based on discussions with the FDA, we and JRD have carefully considered potential new study designs to
determine the effects of ESAs on survival and tumor outcomes in anemic patients with metastatic cancer receiving concomitant
myelosuppressive chemotherapy. Based on those discussions, we are conducting a randomized, double-blind, placebo-controlled,
phase 3 non-inferiority study evaluating overall survival when comparing advanced non small cell lung cancer (NSCLC) patients
on Aranesp® to patients receiving placebo (Study '782) as part of our Aranesp® pharmacovigilance program. In addition, JRD’s
EPO-ANE-3010 study in breast cancer is ongoing. Both studies are designated by the FDA as PMR clinical trials. For the nephrology
setting, we have been engaged in ongoing discussions with the FDA regarding additional PMRs to explore alternative ESA dosing
strategies in CKD patients on dialysis and not on dialysis. In July 2012 we initiated study '226 to evaluate Aranesp® use in CKD
patients not on dialysis. We expect to discuss further with the FDA a potential study in CKD patients on dialysis.
In January 2013, we announced the top-line results of the phase 3 Aranesp® RED-HF® (Reduction of Events With Darbepoetin
Alfa in Heart Failure) Trial. The trial was initiated in 2006, and a total of 2,278 patients with symptomatic systolic heart failure
and anemia (Hb levels ranging from 9.0-12.0 g/dL) were randomized to receive either treatment with Aranesp® to achieve a target
Hb of at least 13.0 g/dL (not to exceed 14.5 g/dL), or placebo. The study did not meet its primary endpoint of reducing the composite
endpoint of time to death from any cause or first hospital admission for worsening heart failure. There were no new safety findings
identified in the study. These summary results will be followed by full efficacy and safety analyses, which will be shared and
discussed with global regulatory agencies and submitted for presentation at an upcoming medical meeting.
Adverse events or results of any of these studies could further affect product labeling, healthcare provider prescribing
behavior, regulatory or private healthcare organization medical guidelines and/or reimbursement practices related to Aranesp® or
EPOGEN®.
Aranesp® (darbepoetin alfa)
We were granted an exclusive license by K-A to manufacture and market Aranesp® in the United States, all European
countries, Canada, Australia, New Zealand, Mexico, all Central and South American countries and certain countries in Central
Asia, Africa and the Middle East.
We market Aranesp® primarily in the United States and Europe, and it was launched in 2001 in both regions. It is indicated
for the treatment of anemia associated with CKD (in both patients on dialysis and patients not on dialysis) and also for the treatment
of anemia due to concomitant chemotherapy in patients with non-myeloid malignancies.
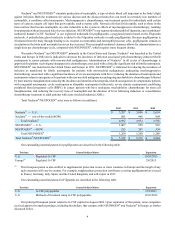
Total Aranesp® sales were as follows (in millions):
2012 2011 2010
Aranesp® — U.S. $ 782 $ 986 $ 1,103
Aranesp® — ROW 1,258 1,317 1,383
Total Aranesp®$ 2,040 $ 2,303 $ 2,486
Our outstanding material patents for darbepoetin alfa are described in the following table.
Territory General Subject Matter Expiration
U.S. Glycosylation analogs of erythropoietin proteins 5/15/2024
Europe(1) Glycosylation analogs of erythropoietin proteins 8/16/2014