Amgen 2012 Annual Report Download - page 12
Download and view the complete annual report
Please find page 12 of the 2012 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
5
Our outstanding material U.S. patents for Filgrastim (NEUPOGEN®) expire in December 2013. We expect to face competition
in the United States beginning in the fourth quarter of 2013, which may have a material adverse impact over time on future sales
of NEUPOGEN® and, in turn, Neulasta®. See discussion of Teva below.
Any products or technologies that are directly or indirectly successful in treating neutropenia associated with chemotherapy,
for bone marrow and PBPC transplant patients, severe chronic neutropenia and AML could negatively impact Neulasta® and/or
NEUPOGEN® sales. Neulasta® and/or NEUPOGEN® sales may also be impacted by increases or decreases in the use of
myelosuppressive chemotherapy, which may result from changes in the number of patients being treated, changes to treatment
protocols or the introduction of new cancer treatments that may not be myelosuppressive. Further, NEUPOGEN® competes with
Neulasta® in the United States and Europe, and NEUPOGEN® sales have been adversely impacted by conversion to Neulasta®,
which we believe is substantially complete.
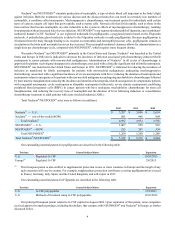
The following table reflects companies and their currently marketed products that compete with Neulasta® and/or
NEUPOGEN® in the United States and Europe in the supportive cancer care setting. The table below and the following discussion
of competitor marketed products and products in development may not be exhaustive.
Territory Competitor Marketed Product Competitor
U.S. Leukine®Bayer HealthCare Pharmaceuticals (Bayer)
Europe Granocyte®Chugai Pharmaceuticals Co., Ltd./Sanofi-Aventis (Sanofi)
Europe Ratiograstim®(1)/Biograstim®(1) ratiopharm GmbH (ratiopharm)(2)/CT Arzneimittel GmbH (CT Arzneimittel)
Europe Tevagrastim®(1) Teva Pharmaceutical Industries Ltd. (Teva Pharmaceutical)
Europe Zarzio®(1)/Filgrastim Hexal®(1) Sandoz GmbH (Sandoz)/Hexal Biotech Forschungs GmbH (Hexal)
Europe Nivestim®(1) Hospira Inc. (Hospira)
(1) Approved via the EU biosimilar regulatory pathway.
(2) A subsidiary of Teva Pharmaceutical.
In August 2012, the FDA approved Sicor Biotech's (Teva Corporation) tbo-filgrastim product to reduce the time that certain
patients receiving cancer chemotherapy experience severe neutropenia. The approval was on the basis of a full BLA rather than
under the FDA's new biosimilar approval pathway. This drug may compete with NEUPOGEN® subject to the terms of the injunction
and settlement agreement discussed below.
In November 2009, Teva Pharmaceutical filed a declaratory judgment action against us alleging that certain of our
NEUPOGEN® patents are invalid and not infringed by its tbo-filgrastim product, and in January 2010, we filed an answer and
counterclaims seeking a declaratory judgment that our patents are valid and infringed. In July 2011, we announced that the U.S.
District Court in Pennsylvania entered final judgment and a permanent injunction against Teva Pharmaceutical and Teva
Pharmaceuticals USA, Inc. (together defined as Teva) prohibiting them from infringing our patents relating to human G-CSF
polypeptides and methods of treatment. The court’s injunction extends until November 10, 2013, after which date Teva will no
longer be prohibited by the injunction from selling its tbo-filgrastim product in the United States. Teva also agreed not to sell
balugrastim, a long-acting product candidate, in the United States before November 10, 2013, unless it first obtains a final court
decision that our patents are not infringed by balugrastim. Pursuant to the parties’ settlement, the launch date for either product
could be sooner if certain unexpected events occur: a third party launches a similar G-CSF polypeptide product and we fail to sue
that third party, or the patents are held invalid or unenforceable in a final court decision in an action brought by a third party.
Several companies have short-acting filgrastim product candidates in phase 3 clinical development, including:
• Merck & Company, Inc. (Merck) (MK-4214)
• Intas/Apotex Inc. (Neukine)
• Reliance Life Sciences Pvt. Ltd. (ReliGrast)
• Biocon Ltd./Celgene Corporation (Celgene) (Nufil)
In addition, several companies have long-acting filgrastim product candidates in phase 3 clinical development, including:
• Teva Pharmaceutical (balugrastim and Lonquex)
• Sandoz (LA-EP2006)
• Intas/Apotex Inc. (Neupeg)