Amgen 2012 Annual Report Download - page 16
Download and view the complete annual report
Please find page 16 of the 2012 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
9
(1) This European patent is also entitled to supplemental protection in one or more countries in Europe and the length of any
such extension will vary by country. For example, supplementary protection certificates covering darbepoetin alfa have
issued in France, Germany, Italy, Spain, and the United Kingdom, and will expire in 2016.
Our principal European patent related to epoetin alfa expired in December 2004. Although we do not market EPOGEN® in
Europe, upon expiration of this patent, some companies received approval to market products, including biosimilars, that compete
with Aranesp® in Europe, as further discussed below.
Any products or technologies that are directly or indirectly successful in addressing anemia associated with chemotherapy
and/or renal failure could negatively impact Aranesp® sales. In the United States, Aranesp® competes with EPOGEN®, primarily
in the U.S. hospital dialysis clinic setting.
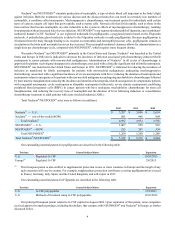
The following table reflects companies and their currently marketed products that compete with Aranesp® in the United
States and Europe in the supportive cancer care and nephrology segments, unless otherwise indicated. The table below and the
following discussion of competitor products in development may not be exhaustive.
Territory Competitor Marketed Product Competitor
U.S. PROCRIT®(1) Janssen(2)
Europe EPREX®/ERYPO®Janssen-Cilag(2)
Europe NeoRecormon®Roche
Europe Retacrit™(3)/Silapo®(3) Hospira/Stada Arzneimittel AG
Europe Binocrit®(3)/epoetin alfa Hexal®(3)/Abseamed®(3) Sandoz/Hexal/Medice Arzneimittel Pütter GmbH & Co. KG
Europe MIRCERA®(4) Roche
Europe Eporatio®/Biopoin® ratiopharm (5)/CT Arzneimittel
(1) PROCRIT® competes with Aranesp® in the supportive cancer care and pre-dialysis settings.
(2) A subsidiary of J&J.
(3) Approved via the EU biosimilar regulatory pathway.
(4) Competes with Aranesp® in the nephrology segment only. Pursuant to a December 2009 settlement agreement between
Amgen and Roche, Roche is allowed to begin selling MIRCERA® in the United States in mid-2014 under terms of a limited
license agreement. MIRCERA® has been approved by the FDA for the treatment of anemia associated with chronic renal
failure (CRF) in patients on and not on dialysis.
(5) A subsidiary of Teva Pharmaceutical.
Several companies have short-acting ESA candidates in late stage clinical development, some of which may be pursued as
biosimilars with U.S.-sourced epoetin alfa as the comparator product, including:
• APOTEX Inc. (APO-EPO)
• Hospira (Retacrit)
• Sandoz (HX-575)
EPOGEN® (epoetin alfa)
We were granted an exclusive license to manufacture and market EPOGEN® in the United States under a licensing agreement
with K-A. We have retained exclusive rights to market EPOGEN® in the United States for dialysis patients. We granted Ortho
Pharmaceutical Corporation, a subsidiary of J&J (which has assigned its rights under the Product License Agreement to Janssen),
a license to commercialize recombinant human erythropoietin as a human therapeutic in the United States in all indications other
than dialysis.
We market EPOGEN® in the United States and it was launched in 1989. EPOGEN® is indicated to treat a lower than normal
number of red blood cells (anemia) caused by CKD in patients on dialysis to lessen the need for red blood cell transfusions.