Amgen 2012 Annual Report Download - page 19
Download and view the complete annual report
Please find page 19 of the 2012 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
12
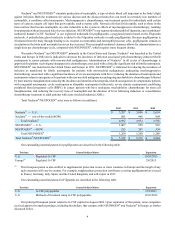
Our outstanding material patents for denosumab are described in the following table.
Territory General Subject Matter Expiration(1)
U.S. RANKL antibodies; and methods of use 12/22/2017
U.S. Methods of treatment 11/11/2018
U.S. RANKL antibodies including sequences 2/19/2025
U.S. Nucleic acids encoding RANKL antibodies, and methods of producing RANKL antibodies 11/30/2023
Europe RANKL antibodies 12/22/2017
Europe Medical use of RANKL antibodies 4/15/2018
Europe RANKL antibodies including epitope binding 2/23/2021
Europe RANKL antibodies including sequences 6/25/2022
(1) In some cases, these patents may be entitled to patent term extension in the United States or supplemental protection in one
or more countries in Europe and the length of any such extension will vary by country. For example, supplementary protection
certificates covering denosumab have issued in France, Italy and Spain, and will expire in 2025.
Other Marketed Products
Our other marketed products include Sensipar®/Mimpara®
(cinacalcet), Vectibix® (panitumumab) and Nplate® (romiplostim).
Sensipar®/Mimpara® (cinacalcet)
Sensipar® is our registered trademark in the United States and Mimpara® is our registered trademark in Europe for cinacalcet,
our small molecule medicine used in treating CKD patients on dialysis who produce too much parathyroid hormone (PTH), a
condition known as secondary hyperparathyroidism. In 2004, Sensipar®/Mimpara® was approved in the United States and Europe
for the treatment of secondary hyperparathyroidism in CKD patients on dialysis and for the treatment of hypercalcemia in patients
with parathyroid carcinoma. In 2008, Mimpara® was approved in Europe for the reduction of hypercalcemia in patients with
primary hyperparathyroidism (PHPT) where a parathyroidectomy is not clinically appropriate or is contraindicated. In 2011,
Sensipar® was approved in the United States for the treatment of severe hypercalcemia in patients with PHPT who are unable to
undergo parathyroidectomy. We market Sensipar® primarily in the United States and Mimpara® primarily in Europe.
As previously discussed, CMS’ s Final Rule on Bundling in Dialysis became effective on January 1, 2011, and provides a
single payment for all dialysis services. Oral drugs without intravenous equivalents, such as Sensipar® and phosphate binders,
will continue to be reimbursed separately under the Medicare Part D benefit until they are included in the bundled-payment system,
which was delayed by Congress from 2014 to 2016 in connection with the passage in January 2013 of the American Taxpayer
Relief Act (ATRA). Inclusion in the bundled-payment system may reduce utilization of these oral drugs and have an adverse
impact on our sales. See Reimbursement.
In November 2012, we presented at ASN's Kidney Week the results of the phase 3 E.V.O.L.V.E™ trial. As previously reported,
the primary analysis showed that the trial did not reach its primary endpoint (time to composite event comprising all-cause mortality
or first non-fatal cardiovascular event, including myocardial infarction, hospitalization for unstable angina, heart failure or
peripheral vascular event) in the intent-to-treat analysis. See Significant Developments in our Quarterly Report on Form 10-Q for
the period ended June 30, 2012.
Total Sensipar®/Mimpara® sales were as follows (in millions):
2012 2011 2010
Total Sensipar®/Mimpara®$ 950 $ 808 $ 714