Amgen 2012 Annual Report Download - page 13
Download and view the complete annual report
Please find page 13 of the 2012 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
6
Enbrel® (etanercept)
ENBREL is our registered trademark for etanercept, our TNF receptor fusion protein that inhibits the binding of TNF to its
receptors, which can result in a significant reduction in inflammatory activity. TNF is one of the chemical messengers that help
regulate the inflammatory process. When the body produces too much TNF, it overwhelms the immune system’s ability to control
inflammation of the joints or of psoriasis-affected skin areas. ENBREL binds certain TNF molecules before they can trigger
inflammation.
ENBREL was launched in the United States in November 1998 and in Canada in March 2001. ENBREL is indicated for the
treatment of adult patients with the following conditions: moderate to severe active RA; chronic moderate to severe plaque psoriasis
patients who are candidates for systemic therapy or phototherapy; active psoriatic arthritis; and active ankylosing spondylitis. It
is also indicated for reducing signs and symptoms of moderately to severely active polyarticular juvenile idiopathic arthritis in
patients ages two and older.
We market ENBREL under a collaboration agreement with Pfizer Inc. (Pfizer) in the United States and Canada, which
expires October 31, 2013. (See Business Relationships — Pfizer Inc.) The rights to market and sell ENBREL outside the United
States and Canada are reserved to Pfizer.
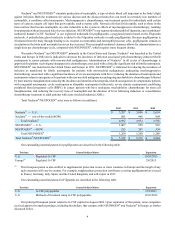
Total ENBREL sales were as follows (in millions):
2012 2011 2010
Total ENBREL $ 4,236 $ 3,701 $ 3,534
Our outstanding material patents for etanercept are described in the following table.
Territory General Subject Matter Expiration
U.S. Methods of treating psoriasis 8/13/2019
U.S. Aqueous formulation and methods of treatment using the formulation(1) 6/8/2023
U.S. Fusion protein, and pharmaceutical compositions 11/22/2028
U.S. DNA encoding fusion protein, and methods of making fusion protein 4/24/2029
(1) This formulation patent relates to the currently approved liquid formulation of ENBREL, which formulation accounts for
the majority of ENBREL sales in the United States. However, ENBREL is also sold as an alternative lyophilized formulation
that requires reconstituting before it can be administered to the patient.
Any products or technologies that are directly or indirectly successful in treating rheumatologic conditions, which includes
moderate to severe RA; moderate to severe polyarticular juvenile idiopathic arthritis; ankylosing spondylitis and psoriatic arthritis;
and dermatologic conditions, which includes moderate to severe plaque psoriasis, could negatively impact ENBREL sales. Certain
of the treatments for these indications include generic methotrexate and other products.
The following table reflects companies and their currently marketed products that compete with ENBREL in the United
States and Canada in the inflammatory disease setting. The table below and the following discussion of competitor marketed
products and products in development may not be exhaustive.
Territory Therapeutic Area
Competitor
Marketed
Product Competitor
U.S. & Canada Rheumatology & Dermatology REMICADE®Janssen Biotech, Inc. (Janssen)(1)/Merck
U.S. & Canada Rheumatology & Dermatology HUMIRA®Abbott Laboratories (Abbott) (2)
U.S. & Canada Rheumatology & Dermatology Simponi®Janssen (1)
U.S. & Canada Rheumatology Cimzia®UCB/Nektar Therapeutics (Nektar)
U.S. & Canada Rheumatology Orencia®Bristol-Myers Squibb Company (BMS)
U.S. & Canada Rheumatology Rituxan®F. Hoffmann-La Roche Ltd (Roche)
U.S. Rheumatology Actemra®Roche
U.S. & Canada Dermatology Stelara®Janssen (1)
U.S. Rheumatology Xeljanz®Pfizer