Amgen 2012 Annual Report Download - page 14
Download and view the complete annual report
Please find page 14 of the 2012 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.7
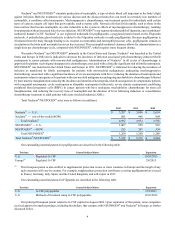
(1) A subsidiary of Johnson & Johnson (J&J).
(2) In January 2013, Abbott announced that it completed the separation of its research-based pharmaceuticals business, which
became AbbVie, Inc. (AbbVie), a new independent biopharmaceutical company which now owns the rights to this product.
In November 2012, the FDA approved Pfizer's Xeljanz® (tofacitinib), an oral treatment for patients with moderate to severe
RA who have had an inadequate response or intolerance to methotrexate. In addition, a number of companies have product
candidates in phase 3 clinical development which may compete with ENBREL in the future, including:
• Celgene (apremilast), in both psoriasis and psoriatic arthritis
• AstraZeneca and Rigel Pharmaceuticals Inc. (fostamatinib) in RA
• Eli Lilly and Company (Eli Lilly) (ixekizumab) for moderate to severe plaque psoriasis
• UCB/Nektar’s Cimzia® in psoriatic arthritis
• Janssen’s Simponi® IV in RA and Stelara® in psoriatic arthritis
• Roche’s Actemra® SC in RA
ESAs
Aranesp® and EPOGEN® are our registered trademarks for darbepoetin alfa and epoetin alfa, respectively, both of which
are proteins that stimulate red blood cell production in a process known as erythropoiesis. Red blood cells transport oxygen to all
cells of the body. Without adequate amounts of a protein called erythropoietin, the red blood cell count is reduced. A deficient red
blood cell count can result in anemia, a condition in which insufficient oxygen is delivered to the body’s organs and tissues. Anemia
can be associated with chronic kidney disease (CKD) in patients either on or not on dialysis. Individuals with CKD may suffer
from anemia because they do not produce sufficient amounts of erythropoietin, which is normally produced in healthy kidneys
and stimulates erythropoiesis. Anemia can also result from chemotherapy treatments for patients with non-myeloid malignancies.
ESAs, including ours, have faced and continue to face challenges. For example, based on adverse safety results observed
beginning in late 2006 in various studies, performed by us and by others, that explored the use of ESAs in settings different from
those outlined in the FDA approved label, the product labeling of our ESAs in the United States and the EU has been updated
several times to reflect those safety concerns. In addition, due in part to certain of these developments, reimbursement of our ESAs
in the United States was also revised. These regulatory and reimbursement changes have led to changes in the way ESAs are used
in clinical practice, including by decreasing the number of patients treated with ESAs as well as the average dose and duration of
ESA therapy.
In 2010 and 2011, the FDA and Centers for Medicare & Medicaid Services (CMS) took a number of actions with respect
to the label for and the reimbursement of ESAs:
• Effective January 1, 2011, CMS implemented the Final Rule on Bundling in Dialysis, providing a single payment for
all dialysis services (with the exception of oral drugs without intravenous equivalents).
• In June 2011, the FDA approved ESA label changes impacting both patients on dialysis and those not on dialysis. While
the previous label language specified a hemoglobin (Hb) target range of 10-12 grams per deciliter (g/dL) for patients
in both populations, the new label advises physicians treating patients on dialysis to initiate ESA therapy when the Hb
level is less than 10 g/dL and to reduce or interrupt the dose when the Hb approaches or exceeds 11 g/dL. For CKD
patients not on dialysis receiving ESA treatment, the new label advises physicians to initiate ESA therapy when the Hb
level is less than 10 g/dL and to reduce or interrupt the dose when the Hb exceeds 10 g/dL.
• In November 2011, CMS finalized a rule to update various provisions of its bundled-payment system for dialysis services
and the related end stage renal disease (ESRD) Quality Incentive Program (QIP). The final rule eliminated for payment
year 2013 and beyond the QIP's measure that tracks the percent of a provider's Medicare patients with a Hb level below
10 g/dL.
• In June 2010, CMS opened a National Coverage Analysis (NCA) to examine the use of ESAs to manage anemia in
patients with CKD and dialysis-related anemia. Following further analysis, in June 2011, CMS issued a Final Decision
Memorandum (FDM) in which it determined that it would not issue a National Coverage Determination (NCD) at that
time for ESAs for treatment of anemia in adults with CKD. In the absence of an NCD, Local Coverage Determinations
(LCDs) may be made by regional contractors called Medicare Administrative Contractors (MACs). Since CMS issued
their FDM, three MACs have issued a revised LCD relating to anemia in patients with CKD not on dialysis. These three
MACs provide ESA coverage no more restrictive than the revised label.