Amgen 2012 Annual Report Download
Download and view the complete annual report
Please find the complete 2012 Amgen annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Dear Shareholders,
2012 was an exceptional year for Amgen.
We delivered for shareholders, positioned
the company for long-term growth, and
continued to fulfill our mission to serve
patients. Revenues rose 11 percent to
$17.3 billion. Adjusted earnings per share
increased 22 percent to $6.51.* Total
shareholder return in 2012 was 36 percent,
outperforming the S&P 500 and our peer
group. Our performance in 2012 reflects
strength across our product portfolio, effective
commercial execution, commitment to
operational efficiency, dedication of staff, and
sound capital allocation decisions. Building on
this success, we entered 2013 with momentum
and confidence in our ability to execute our
long-term strategy of reaching more patients
in more markets around the world.
Delivering for Shareholders
More than a year ago, we made a commitment
to return significant capital to shareholders
in the form of dividends and share buybacks,
and we have delivered on that promise. In
early 2013, we completed the $10-billion
stock repurchase program announced in
October 2011. Since January 2011, we have
repurchased more than 20 percent of our
outstanding shares. In addition, since the
initiation of our first dividend in July 2011,
we have raised the dividend twice over the
previous quarterly amount by an average of
30 percent.
Continued Product Momentum
Amgen’s product sales grew 9 percent in
2012. Sales growth was led by Enbrel®
(etanercept), with solid contributions from
Prolia® (denosumab), XGEVA® (denosumab),
Sensipar® (cinacalcet), Nplate® (romiplostim),
and Vectibix® (panitumumab). In 2012, two of
our products achieved more than $4 billion in
sales; three other products achieved more
than $1 billion in sales, as did our recently
launched denosumab franchise. We also saw
our European business continue to grow in a
challenging economic environment.
Our products continue to show strong
opportunities for growth. In terms of value,
ENBREL remains the leading biologic in the
fast-growing rheumatology and dermatology
segments, with a proven track record. In addition,
by the end of 2013, the profit share we have in
place with Pfizer Inc. for ENBREL transitions to
a significantly lower royalty. As a result, the
contributions to Amgen’s profitability from ENBREL
will grow substantially starting in 2014.
There are also continued unmet medical needs
that can be addressed by Neulasta®
(pegfilgrastim)/NEUPOGEN® (Filgrastim), including
many breast cancer patients undergoing
myelosuppressive therapy associated with a
clinically significant risk of febrile neutropenia.
We continue to launch XGEVA® in Europe and
expand access for Prolia® in the U.S. We expect
that EPOGEN® (epoetin alfa) and Aranesp®
(darbepoetin alfa) will remain important therapies
due to a long history of use by physicians in the
treatment of anemia. Sensipar®/Mimpara®,
indicated for the treatment of secondary
hyperparathyroidism in patients with chronic
kidney disease who are on dialysis, is on track
to exceed $1 billion in sales in 2013.
Emerging Late-Stage Pipeline
In 2012, we made clear progress in advancing
our pipeline. Our pipeline focuses on innovative,
biological targets and molecules that address
serious illnesses and areas of high unmet medical
need; and this reflects our strategic focus of
unlocking the potential of biology for patients. At
Amgen, we take a “biology first” approach, which
means that we examine the fundamental
mechanisms of human biology to unravel the
complexities of disease in order to interdict them
with our medicines. From 2013 to 2016, we
expect to generate pivotal data for eight of our
pipeline molecules. As of early 2013, we have
six investigational molecules in phase 3 trials
and five investigational molecules in phase 2 trials
to treat diseases in areas including cardiovascular
disease, bone disease, inflammation, nephrology,
oncology, and neuroscience.
AMG 145 is one of many therapies in our pipeline
that shows great promise. Consistent with
Amgen’s strategic focus on combating serious
Letter to
Shareholders
*“Adjusted” earnings per share is a non-GAAP financial measure. See back page for reconciliation to U.S. generally accepted accounting principles (GAAP).
Robert A. Bradway, Chairman and Chief Executive Officer, Amgen Inc.
Table of contents
-
Page 1
... starting in 2014. There are also continued unmet medical needs that can be addressed by Neulasta ® ® ® ® (pegï¬lgrastim)/NEUPOGEN® (Filgrastim), including many breast cancer patients undergoing myelosuppressive therapy associated with a clinically signiï¬cant risk of febrile neutropenia... -
Page 2
... delivering them to patients. The manufacturing process for We recently advanced our strategy for global expansion on a number of fronts and will continue to grow internationally. As of 2012, we were selling our products in 56 countries and expect to reach 75 countries by 2015. Plans are under way... -
Page 3
... Amgen began the execution of a planned leadership transition with the retirement of Kevin Sharer, Amgen's third chairman and chief executive officer. I would like to recognize the extraordinary leadership of my friend and predecessor. Kevin retired from Amgen after a 20-year career with the company... -
Page 4
...the growth of our business and other ï¬nancial metrics; expected clinical or regulatory results or practices; development of Amgen's product candidates, including anticipated regulatory ï¬lings; our manufacturing capabilities; and planned international expansion. Forward-looking statements involve... -
Page 5
...OF THE SECURITIES EXCHANGE ACT OF 1934 Commission file number 000-12477 Amgen Inc. (Exact name of registrant as specified in its charter) Delaware (State or other jurisdiction of incorporation or organization) One Amgen Center Drive, Thousand Oaks, California (Address of principal executive offices... -
Page 6
... shares of common stock held by directors and executive officers at June 30, 2012. Exclusion of shares held by any person should not be construed to indicate that such person possesses the power, directly or indirectly, to direct or cause the direction of the management or policies of the registrant... -
Page 7
... Marketed Products Marketing and Distribution Reimbursement Manufacturing, Distribution and Raw Materials Government Regulation Research and Development and Selected Product Candidates Business Relationships Human Resources Executive Officers of the Registrant Geographic Area Financial Information... -
Page 8
...of our principal products as well as most of our product candidates. We operate a number of commercial and/or clinical manufacturing facilities, and our primary manufacturing facilities are located in the United States, Puerto Rico and the Netherlands. See Item 2. Properties. Drug development in our... -
Page 9
.... In March 2012, we entered into a collaboration agreement with AstraZeneca to jointly develop and commercialize certain monoclonal antibodies from Amgen's clinical inflammation portfolio including brodalumab, AMG 139, AMG 157, AMG 181 and AMG 557. The agreement covers the worldwide development and... -
Page 10
... (Filgrastim) We were granted an exclusive license to manufacture and market Neulasta® and NEUPOGEN® in the United States, Europe, Canada and Australia under a licensing agreement with Kirin-Amgen, Inc. (K-A), a joint venture between Kirin Holdings Company, Limited (Kirin), and Amgen. See Business... -
Page 11
... dosing. We market Neulasta® and NEUPOGEN® primarily in the United States and Europe. Neulasta® was launched in the United States and Europe in 2002 and is indicated to decrease the incidence of infection associated with chemotherapy-induced febrile neutropenia in cancer patients with non-myeloid... -
Page 12
... table reflects companies and their currently marketed products that compete with Neulasta® and/or NEUPOGEN® in the United States and Europe in the supportive cancer care setting. The table below and the following discussion of competitor marketed products and products in development may not... -
Page 13
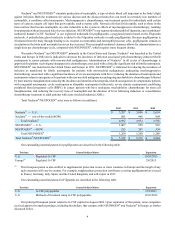
...to market and sell ENBREL outside the United States and Canada are reserved to Pfizer. Total ENBREL sales were as follows (in millions): 2012 2011 2010 Total ENBREL $ 4,236 $ 3,701 $ 3,534 Our outstanding material patents for etanercept are described in the following table. Territory General... -
Page 14
... number of companies have product candidates in phase 3 clinical development which may compete with ENBREL in the future, including ESAs Aranesp® and EPOGEN® are our registered trademarks for darbepoetin alfa and epoetin alfa, respectively, both of which are proteins that stimulate red blood cell... -
Page 15
...medical guidelines and/or reimbursement practices related to Aranesp® or EPOGEN®. Aranesp® (darbepoetin alfa) We were granted an exclusive license by K-A to manufacture and market Aranesp® in the United States, all European countries, Canada, Australia, New Zealand, Mexico, all Central and South... -
Page 16
... table reflects companies and their currently marketed products that compete with Aranesp® in the United States and Europe in the supportive cancer care and nephrology segments, unless otherwise indicated. The table below and the following discussion of competitor products in development may not... -
Page 17
... certain stabilizers Cells that make certain levels of erythropoietin 8/20/2013 8/20/2013 9/24/2014 5/26/2015 Any products or technologies that are directly or indirectly successful in addressing anemia associated with renal failure could negatively impact EPOGEN® sales. In the United States, as... -
Page 18
... Competitor Marketed Product Competitor U.S. & Europe U.S. & Europe (1) Zometa®(1) Aredia®(2) Novartis AG (Novartis) Novartis Novartis has indicated that patent protection on the active ingredient for Zometa® will expire in 2013 in the United States. At such time, we expect that generic... -
Page 19
... parathyroidectomy. We market Sensipar® primarily in the United States and Mimpara® primarily in Europe. As previously discussed, CMS's Final Rule on Bundling in Dialysis became effective on January 1, 2011, and provides a single payment for all dialysis services. Oral drugs without intravenous... -
Page 20
... sales. The following table reflects companies and their currently marketed products that compete with Sensipar® in the United States and with Mimpara® in Europe in the nephrology segment for patients with CKD on dialysis and may not be exhaustive. Territory Competitor Marketed Product Competitor... -
Page 21
... counts. We were granted an exclusive license by K-A to manufacture and market Nplate® in the United States, all European countries, Canada, Australia, New Zealand, Mexico, all Central and South American countries and certain countries in Central Asia, Africa and the Middle East. In February 2009... -
Page 22
... Product Competitor U.S. Europe Promacta Revolade® ® GSK GSK Marketing and Distribution We maintain sales and marketing forces primarily in the United States, Europe and Canada to support our currently marketed products. We have also entered into agreements with third parties to assist... -
Page 23
..., which are national policy determinations granting, limiting or excluding Medicare coverage for specific medical items or services applicable throughout the United States. In the absence of a relevant NCD, Medicare coverage determinations for a particular medical item or service are left to MACs... -
Page 24
... main pathway by which Americans receive private health insurance. Many employers provide health insurance as part of employees' benefit packages. Insurance plans are administered by private companies - both for-profit and not-for-profit - and some companies are self-insured (i.e., they pay directly... -
Page 25
...under the Public Health Service (PHS) drug pricing program to eligible community health clinics and other entities that receive health services grants from the PHS, as well as hospitals that serve a disproportionate share of Medicare and Medicaid beneficiaries. We also make our products available to... -
Page 26
... level (FPL), from 100% of the FPL. This provision becomes effective January 1, 2014. Impact of Budget Control Act on U.S. Reimbursement The Budget Control Act of 2011, signed into law in the United States in August 2011, mandated a 2% reduction in government payments for all Medicare services... -
Page 27
... into vials or syringes. Finally, in the finish process, our products are packaged for distribution. We operate a number of commercial and/or clinical manufacturing facilities, and our primary facilities are located in the United States, Puerto Rico and the Netherlands. (See Item 2. Properties.) We... -
Page 28
... at the Puerto Rico facility, we may not be able to supply these products or, at the Thousand Oaks facility, we may not be able to continue our clinical trials. Distribution We operate distribution centers in the United States, principally in Kentucky, California and the Netherlands for worldwide... -
Page 29
... the Public Health Service Act, the FDCA and the regulations promulgated thereunder, as well as other federal and state statutes and regulations govern, among other things, the raw materials and components used in the production, research, development, testing, manufacture, quality control, labeling... -
Page 30
...to conduct further clinical trials to provide additional information on our marketed products' safety and efficacy. Those additional trials may include studying doses or schedules of administration different from those used in previous studies, use in other patient populations or other stages of the... -
Page 31
... region. New Innovation Provisions Available to Regulatory Agencies Reviewing Drug Applications. In the United States, the FDA may grant accelerated approval status to products that treat serious or life-threatening illnesses and that provide meaningful therapeutic benefits to patients over existing... -
Page 32
... various State False Claims Acts. In connection with entering into the settlement agreement, Amgen also entered into a corporate integrity agreement with the Office of the Inspector General of the U.S. Department of Health and Human Services that requires Amgen to maintain its corporate compliance... -
Page 33
... United States and in the United Kingdom, as well as smaller research centers and development facilities globally. See Item 2. Properties. We conduct clinical trial activities using both our internal staff and third-party contract clinical trial service providers. To increase the number of patients... -
Page 34
...2 diabetes Inflammatory bowel disease Secondary hyperparathyroidism in patients with CKD receiving dialysis Schizophrenia Acute lymphoblastic leukemia (ALL) Non-Hodgkin's Lymphoma (NHL) Inflammatory diseases Heart failure RA Various cancer types Squamous cell head and neck cancer Giant cell tumor of... -
Page 35
... and proper dose ranges of a product candidate in a small number of human subjects. clinical trials investigate side effect profiles and efficacy of a product candidate in a large number of patients who have the disease or condition under study. clinical trials investigate the safety and efficacy of... -
Page 36
... the human cell surface CaSR. It is being investigated as a treatment for secondary hyperparathyroidism in patients with CKD receiving dialysis. We completed two phase 2 studies in 2012. Phase 3 initiation is planned in 2013. AMG 747 AMG 747 is a small molecule inhibitor of glycine transporter type... -
Page 37
... Omecamtiv mecarbil Omecamtiv mecarbil is a small molecule activator of cardiac myosin. It is being investigated for the treatment of heart failure. We are developing this product in collaboration with Cytokinetics, Inc. A phase 2 study of an intravenous formulation of omecamtiv mecarbil in patients... -
Page 38
... the United States, Europe, Canada, Australia, New Zealand, Mexico, all Central and South American countries and certain countries in Central Asia, Africa and the Middle East, and (iii) recombinant human erythropoietin in the United States. We currently market pegfilgrastim, G-CSF, darbepoetin alfa... -
Page 39
...marketing plan, product pricing and the establishment of a brand team. Amgen and Pfizer share in the agreed-upon selling and marketing expenses approved by the joint management committee. We currently pay Pfizer a percentage of annual gross profits on our ENBREL sales in the United States and Canada... -
Page 40
... ESAs used in providing dialysis services in the United States and Puerto Rico. The agreement may be terminated by either party before expiration of its term in the event of certain breaches of the agreement by the other party. Human Resources As of December 31, 2012, Amgen had approximately 18,000... -
Page 41
...Mr. McNamee held human resources positions at GE. Ms. Cynthia M. Patton, age 51, became Senior Vice President and Chief Compliance Officer in October 2012. Ms. Patton joined the Company in 2005. From September 2010 to October 2012, Ms. Patton was Vice President, Law. From July 2005 to September 2010... -
Page 42
... pay for such products, which could materially and adversely affect sales of our products. Private payers also continue to seek to reduce their costs. Insurance plans administered by private companies frequently adopt their own payment or reimbursement reductions. Consolidation among managed care... -
Page 43
... for our products or the level of coverage and reimbursement available when healthcare providers prescribe our products, they could have a material adverse effect on the sales of our products, our business and results of operations. We also face risks relating to the reporting of pricing data that... -
Page 44
... the United States or the impact such actions will have on our business, such reductions in price and/or the coverage and reimbursement for our products could have a material adverse effect on the sales of our products, our business and results of operations. Additional initiatives addressing the... -
Page 45
... or regulatory policies affecting our business could occur, such as efforts to reform medical device regulation or the pedigree requirements for medical products or implement new requirements for combination products, and whether such changes could have a material adverse effect on our business and... -
Page 46
..., a number of regulatory agencies around the world, including the FDA and the EMA, have initiated programs to directly monitor for safety issues rather than wait for patients, providers or manufacturers to report safety problems with products or medical devices. And at least one private, for-profit... -
Page 47
... as J&J, Pfizer, Glaxo and Takeda) report or fail to effectively report to regulatory agencies side effects or other safety concerns that occur from their use of our products in clinical trials or studies or from marketed use, resulting regulatory action, including monetary fines and other penalties... -
Page 48
... lower cost biosimilars could have a material adverse effect on the sales of our products, our business and results of operations. In addition, as a result of the economic conditions and/or employer decisions regarding the insurance coverage mandate that goes into effect in the United States in 2014... -
Page 49
... in the availability of, or the coverage and/or reimbursement for, in vitro companion diagnostic or drug delivery devices used with our products could have a material adverse effect on our product sales, business and results of operations. Our ESAs continue to be under review and receive scrutiny by... -
Page 50
... approvals in the timeframe needed to execute our product strategies, our business and results of operations could be materially and adversely affected. Additional information on our clinical trials can be found on our website at www.amgen.com. (This website address is not intended to function as... -
Page 51
... private health organization medical guidelines and reimbursement for our products, all of which could have a material adverse effect on our business and results of operations. Clinical trials must be designed based on the current standard of medical care. However in certain diseases, such as cancer... -
Page 52
... United States as a result of biosimilars and downward pressure on our product prices and sales, subject to our ability to enforce our patents. (See Item 7A. Management's Discussion and Analysis of Financial Condition and Results of Operations - Financial Condition, Liquidity and Capital Resources... -
Page 53
... is uncertain or not well-defined. • • • • Several of our product candidates have failed or been discontinued at various stages in the product development process. For example, in June 2004, we announced that the phase 2 study of Glial Cell Lined-Derived Neurotrophic Factor (GDNF... -
Page 54
... products, EPOGEN®, is sold primarily to free-standing dialysis clinics, which have experienced significant consolidation. Two organizations, DaVita and Fresenius Medical Care North America, own or manage a large number of the outpatient dialysis facilities located in the United States and account... -
Page 55
..., natural disaster, or otherwise; degree of compliance with regulatory requirements; changes in forecasts of future demand; timing and actual number of production runs; updating of manufacturing specifications; production success rates and yields; and timing and outcome of product quality testing... -
Page 56
... at our Thousand Oaks, California manufacturing facility; if significant natural disasters or production failures occur at the Puerto Rico facility, we may not be able to supply these products or, at the Thousand Oaks facility, we may not be able to continue our clinical trials. We currently... -
Page 57
... its filgrastim product, upon approval from the FDA, in the United States without a license from us and prior to the expiration of our G-CSF patents. The period of time from inception until resolution of a patent dispute or litigation is subject to the availability and schedule of the court, agency... -
Page 58
...®) to patients who have a very low risk for febrile neutropenia, a position consistent with ASCO's existing guidelines for the use of white blood cell stimulating factors. • In addition, HTA organizations, such as NICE in the UK and the Canadian Agency for Drugs and Technologies in Health, make... -
Page 59
...all potentially applicable foreign regulations and/or other requirements. The development, manufacturing, distribution, pricing, sales, marketing and reimbursement of our products, together with our general operations, are subject to extensive federal and state regulation in the United States and to... -
Page 60
... reputation and business. Third parties may illegally distribute and sell counterfeit versions of our products, which do not meet the exacting standards of our Company's development, manufacturing and distribution processes. Counterfeit medicines pose a significant risk to patient health and safety... -
Page 61
issues and opportunities. Failures or difficulties in integrating the operations of the businesses that we acquire, including their personnel, technology, financial systems, distribution and general business operations and procedures, may affect our ability to grow and may result in us incurring ... -
Page 62
... as of December 31, 2012. For additional information regarding manufacturing initiatives, see Item 1. Business - Manufacturing, Distribution and Raw Materials. Our corporate headquarters are located in Thousand Oaks, California. In addition to the properties listed above, we have undeveloped... -
Page 63
... Oaks, California, manufacturing facility; if significant natural disasters or production failures occur at the Puerto Rico facility, we may not be able to supply these products or, at the Thousand Oaks facility, we may not be able to continue our clinical trials, - We rely on third-party suppliers... -
Page 64
... any filing of the Company under the Securities Act or the Exchange Act, whether made on, before or after the date of this filing and irrespective of any general incorporation language in such filing. Stock repurchase program The Company intends to continue to return capital to stockholders through... -
Page 65
.... Additionally, on December 13, 2012, the Board of Directors declared a quarterly cash dividend of $0.47 per share of common stock, which will be paid on March 7, 2013. We expect to continue to pay quarterly dividends, although the amount and timing of any future dividends are subject to approval by... -
Page 66
... Equity Securities for information regarding cash dividends declared per share of common stock. (1) In 2011, we recorded a $780 million legal settlement charge ($705 million, net of tax) in connection with an agreement in principle to settle allegations related to our sales and marketing practices... -
Page 67
... with accounting principles generally accepted in the United States (GAAP). We are a global biotechnology pioneer that discovers, develops, manufactures and delivers innovative human therapeutics. Our medicines help millions of patients in the fight against cancer, kidney disease, RA, bone disease... -
Page 68
... or collaborate on therapies currently in development by other companies. The discovery and development of safe and effective new products, as well as the development of additional indications for existing products, are necessary for the continued strength of our businesses. Our product lines must... -
Page 69
... manufacturer in Puerto Rico. The excise tax is imposed on the gross intercompany purchase price of the goods and services and is effective for a six-year period beginning in 2011, with the excise tax rate declining in each year (4% in 2011, 3.75% in 2012, 2.75% in 2013, 2.5% in 2014, 2.25% in 2015... -
Page 70
... in Europe, and a decrease in the average net sales price. Our outstanding material U.S. patents for Filgrastim (NEUPOGEN®) expire in December 2013. We expect to face competition in the United States beginning in the fourth quarter of 2013, which may have a material adverse impact over time on... -
Page 71
... changes to the label and to the reimbursement environment that occurred during 2011 (2011 changes). The decrease in ROW Aranesp® sales for 2012 was due primarily to a decrease in the average net sales price. Sequentially, global Aranesp® unit demand was down 5% in the quarter ended December 31... -
Page 72
... with the quarter ended September 30, 2012. XGEVA® also faces increased competition. See Item 1. Business - Marketed Products. Other products Other product sales by geographic region were as follows (dollar amounts in millions): 2012 Change 2011 Change 2010 Sensipar®-U.S. Sensipar®/Mimpara®-ROW... -
Page 73
... well as the costs of obtaining regulatory approval of a product in a new market after approval in either the United States or the EU has been obtained. R&D expense by category was as follows (in millions): 2012 2011 2010 Discovery Research and Translational Sciences Later stage clinical programs... -
Page 74
...for 2011 included primarily a legal settlement charge of $780 million and certain charges related to cost savings initiatives, primarily severance, of $109 million. In 2010, we recorded a $118 million asset impairment charge for our manufacturing operations located in Fremont, California, associated... -
Page 75
... manufacturer in Puerto Rico. The excise tax is imposed on the gross intercompany purchase price of the goods and services and is effective for a six-year period beginning in 2011, with the excise tax rate declining in each year (4% in 2011, 3.75% in 2012, 2.75% in 2013, 2.5% in 2014, 2.25% in 2015... -
Page 76
...operations and existing sources of and access to financing are adequate to satisfy our needs for working capital; capital expenditure and debt service requirements; our plans to pay dividends and repurchase stock; and other business initiatives we plan to strategically pursue, including acquisitions... -
Page 77
... paper to fund our working capital needs. At December 31, 2012 and 2011, we had no amounts outstanding under our commercial paper program. In December 2011, we entered into a $2.5 billion syndicated, unsecured, revolving credit agreement which is available for general corporate purposes or as... -
Page 78
... Capital expenditures, which were associated primarily with manufacturing capacity expansions in Ireland and Puerto Rico, as well as other site developments, totaled $689 million, $567 million and $580 million in 2012, 2011 and 2010, respectively. We currently estimate 2013 spending on capital... -
Page 79
...related to clinical trials) for new and existing products; (iii) capital expenditures; and (iv) open purchase orders for the acquisition of goods and services in the ordinary course of business. Our obligation to pay certain of these amounts may be reduced based on certain future events. Liabilities... -
Page 80
... the aggregate sales deductions charged against product sales in each of the three years ended December 31, 2012. In the United States, we utilize wholesalers as the principal means of distributing our products to healthcare providers, such as physicians or their clinics, dialysis centers, hospitals... -
Page 81
... outside the United States in Puerto Rico pertaining to manufacturing, distribution and other related functions to meet its worldwide product demand. Income from the Company's operations in Puerto Rico is subject to a tax incentive grant that expires in 2020. Our effective tax rate reflects the... -
Page 82
...stage of completion at the acquisition date; projecting the probability and timing of obtaining marketing approval from the FDA and other regulatory agencies for product candidates; estimating the timing of and future net cash flows from product sales resulting from completed products and in-process... -
Page 83
...the capital and credit markets, strong demand for fixed-income instruments led to continued low interest rates on corporate debt issuances during 2012. Short-term interest rates on U.S. Treasury instruments remained near historical lows due to a combination of the Federal Reserve's monetary policies... -
Page 84
..., 2012 and 2011, a hypothetical 20% adverse movement in foreign currency exchange rates compared with the U.S. dollar relative to exchange rates on these dates would not have resulted in a material reduction in the fair value of these contracts on this date and would not result in a material effect... -
Page 85
... in Amgen's Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the SEC's rules and forms, and that such information is accumulated and communicated to Amgen's management, including its Chief Executive Officer and Chief Financial Officer, as... -
Page 86
...with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included... -
Page 87
... AUTHORIZED FOR ISSUANCE UNDER EXISTING EQUITY COMPENSATION PLANS in our Proxy Statement. Security Ownership of Directors and Executive Officers and Certain Beneficial Owners Information about security ownership of certain beneficial owners and management is incorporated by reference from the... -
Page 88
... to Financial Statement Schedules The following Schedule is filed as part of this Annual Report on Form 10-K: Page number II. Valuation and Qualifying Accounts F-51 All other schedules are omitted because they are not applicable, not required or because the required information is included in... -
Page 89
... Agent. (Filed as an exhibit to Form 10-Q for the quarter ended March 31, 1998 on May 13, 1998 and incorporated herein by reference.) Officers' Certificate of Amgen Inc., dated as of May 30, 2007, including forms of the Company's Senior Floating Rate Notes due 2008, 5.85% Senior Notes due 2017 and... -
Page 90
... on Schedule 14A on March 26, 2009 and incorporated herein by reference.) Form of Stock Option Agreement for the Amgen Inc. 2009 Equity Incentive Plan. (As Amended on October 10, 2012.) (Filed as an exhibit to Form 10-Q for the quarter ended September 30, 2012 on November 6, 2012 and incorporated... -
Page 91
... Amgen Nonqualified Deferred Compensation Plan, effective October 12, 2011. (Filed as an exhibit to Form 10-K for the year ended December 31, 2011 on February 29, 2012 and incorporated herein by reference.) Third Amendment to the Amgen Nonqualified Deferred Compensation Plan, effective June 18, 2012... -
Page 92
... Unit Agreement, dated April 27, 2012, between Amgen Inc. and Kevin W. Sharer. (Filed as an exhibit to Form 10-Q for the quarter ended June 30, 2012 on August 8, 2012 and incorporated herein by reference.) Product License Agreement, dated September 30, 1985, and Technology License Agreement, dated... -
Page 93
..., 2005 on March 10, 2006 and incorporated herein by reference.) Credit Agreement, dated as of December 2, 2011, among Amgen Inc., with Citibank, N.A., as administrative agent, JPMorgan Chase Bank, N.A., as syndication agent, Citigroup Global Markets Inc. and J.P. Morgan Securities LLC as joint lead... -
Page 94
...) and Amendment No. 1, effective as of June 9, 2003, to Collaboration and License Agreement between Amgen Inc. and Celltech R&D Limited (with certain confidential information deleted therefrom). Integrated Facilities Management Services Agreement, dated February 4, 2009, between Amgen Inc. and Jones... -
Page 95
Exhibit No. Description 31* 32...Label Linkbase Document. XBRL Taxonomy Extension Presentation Linkbase Document. (* = filed herewith) (** = furnished herewith and not "filed" for purposes of Section 18 of the Securities Exchange Act of 1934, as amended) (+ = management contract or compensatory plan... -
Page 96
... Securities Exchange Act of 1934, the registrant has duly caused this Annual Report to be signed on its behalf by the undersigned, thereunto duly authorized. AMGEN INC. (Registrant) Date: 02/27/2013 By: / S/ JONATHAN M. PEACOCK Jonathan M. Peacock Executive Vice President and Chief Financial Officer... -
Page 97
... 1987 Directors' Stock Option Plan; Registration Statements (Form S-8 Nos. 333-81284 and 333-177868) pertaining to the Amgen Nonqualified Deferred Compensation Plan; Registration Statements (Form S-3 No. 333-56664 and Amendment No. 1 thereto) pertaining to the Amgen Inc. 1997 Special Non-Officer... -
Page 98
... to the consolidated financial statements and schedule of Amgen Inc. and the effectiveness of internal control over financial reporting of Amgen Inc. included in this Annual Report (Form 10-K) for the year ended December 31, 2012. /s/ Ernst & Young LLP Los Angeles, California February 27, 2013 91 -
Page 99
...) Executive Vice President and Chief Financial Officer (Principal Financial Officer) Vice President Finance and Chief Accounting Officer (Principal Accounting Officer) Director Director Director Director Director Director Director Director Director Director Director Director Director 2/27/2013... -
Page 100
..., presents fairly in all material respects the information set forth therein. We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Amgen Inc.'s internal control over financial reporting as of December 31, 2012, based on criteria... -
Page 101
... 31, 2012, 2011 and 2010 (In millions, except per share data) 2012 2011 2010 Revenues: Product sales Other revenues Total revenues Operating expenses: Cost of sales (excludes amortization of certain acquired intangible assets presented separately) Research and development Selling, general and... -
Page 102
..., 2011 and 2010 (In millions) 2012 2011 2010 Net income Other comprehensive income (loss), net of reclassification adjustments and taxes: Foreign currency translation losses Gains (losses) on the effective portion of cash flow hedges Net unrealized gains (losses) on available-for-sale securities... -
Page 103
AMGEN INC. CONSOLIDATED BALANCE SHEETS December 31, 2012 and 2011 (In millions, except per share data) 2012 2011 ASSETS Current assets: Cash and cash equivalents Marketable securities Trade receivables, net Inventories Other current assets Total current assets Property, plant and equipment, net ... -
Page 104
... comprehensive loss, net of tax Dividends Issuance of common stock in connection with the Company's equity award programs Stock-based compensation Tax impact related to employee stock-based compensation Repurchases of common stock Balance at December 31, 2012 994.6 - - $ 26,944 - - $ (4,322... -
Page 105
AMGEN INC. CONSOLIDATED STATEMENTS OF CASH FLOWS Years ended December 31, 2012, 2011 and 2010 (In millions) 2012 2011 2010 Cash flows from operating activities: Net income Depreciation and amortization Stock-based compensation expense Deferred income taxes Property, plant and equipment impairments ... -
Page 106
...significant accounting policies Business Amgen Inc. (including its subsidiaries, referred to as "Amgen," "the Company," "we," "our" or "us") is a global biotechnology pioneer that discovers, develops, manufactures and delivers innovative human therapeutics. Our medicines help millions of patients in... -
Page 107
... as incurred and include primarily salaries, benefits and other staff-related costs; facilities and overhead costs; clinical trial and related clinical manufacturing costs; contract services and other outside costs; information systems' costs and amortization of acquired technology used in R&D with... -
Page 108
... stated at the lower of cost or market. Cost, which includes amounts related to materials, labor and overhead, is determined in a manner that approximates the first-in, first-out method. Cost also includes the Puerto Rico excise tax enacted in 2011 related to our manufacturing operations in Puerto... -
Page 109
...landscape. Adverse clinical trial results, significant delays in obtaining market approval and the inability to bring a product to market could result in the related intangible assets to be partially or fully impaired. We perform an impairment test of goodwill annually and whenever events or changes... -
Page 110
... to as KAI-4169), its lead product candidate, which is in phase 2 clinical development for the treatment of secondary hyperparathyroidism in patients with chronic kidney disease who are on dialysis. The transaction, which was accounted for as a business combination, provides us with an opportunity... -
Page 111
... Inc. (Micromet), a publicly held biotechnology company focused on the discovery, development and commercialization of innovative antibody-based therapies for the treatment of cancer, which became a wholly owned subsidiary of Amgen. This transaction, which was accounted for as a business combination... -
Page 112
... allocated to the acquisition date fair values of assets acquired and liabilities assumed as follows (in millions): Indefinite-lived intangible assets: IPR&D Contract assets Finite-lived intangible assets - Developed technology Goodwill Cash and marketable securities Deferred tax assets (liabilities... -
Page 113
... License Application (BLA) with the FDA; the first commercial sale in each of the United States and the European Union (EU) following receipt of marketing approval, which includes use of the product in specified patient populations; and upon achieving specified levels of sales. The estimated... -
Page 114
... type of award generally determined by the employee's salary grade and performance level. In addition, certain management and professional level employees typically receive RSU grants upon commencement of employment. Prior to 2012, eligible employees also received a grant of stock options annually... -
Page 115
... stock units The grant date fair value of an RSU equaled the closing price of our common stock on the grant date for RSUs granted prior to April 25, 2011, and on and after April 27, 2012. Prior to April 2011, we did not have a policy of paying dividends, and beginning April 27, 2012, RSUs granted... -
Page 116
... is generally three years. The performance goals for the units granted in 2012, 2011 and 2010, which are accounted for as equity awards, are based upon Amgen's stockholder return compared with a comparator group of companies, which are considered market conditions and are reflected in the grant date... -
Page 117
... upon the number of performance units earned multiplied by the closing stock price of our common stock on the last day of the performance period. As of December 31, 2012, there was approximately $179 million of unrecognized compensation cost related to the 2012 and 2011 performance unit grants that... -
Page 118
... liabilities are as follows as of December 31, 2012 and 2011 (in millions): 2012 2011 Deferred income tax assets (1): Expense accruals NOL and credit carryforwards Expenses capitalized for tax Stock-based compensation Deferred revenue Other Total deferred income tax assets Valuation allowance Net... -
Page 119
...with various state and foreign tax authorities for prior tax years. As a result of these developments, we remeasured our UTBs accordingly. During the year ended December 31, 2011, we settled our examination with the Internal Revenue Service (IRS) related to certain transfer pricing tax positions for... -
Page 120
... manufacturer in Puerto Rico. The excise tax is imposed on the gross intercompany purchase price of the goods and services and is effective for a six-year period beginning in 2011, with the excise tax rate declining in each year (4% in 2011, 3.75% in 2012, 2.75% in 2013, 2.5% in 2014, 2.25% in 2015... -
Page 121
...marketing plan, product pricing and the establishment of a brand team. Amgen and Pfizer share in the agreed-upon selling and marketing expenses approved by the joint management committee. We currently pay Pfizer a percentage of annual gross profits on our ENBREL sales in the United States and Canada... -
Page 122
...denosumab for osteoporosis indications in Europe, Australia, New Zealand and Mexico (the Primary Territories). We have retained the rights to commercialize denosumab for all indications in the United States and Canada and for oncology indications in the Primary Territories. Under a related agreement... -
Page 123
... overall survival in patients with advanced non-squamous non small cell lung cancer (NSCLC). In June 2012, the parties materially modified this arrangement such that Amgen licensed all of its rights to motesanib to Takeda which now has control over the worldwide development and commercialization of... -
Page 124
... located in Fremont, California. The transaction closed in March 2011. In connection with the closing of the transaction, BI assumed our obligations under certain of the facility's operating lease contracts and entered into an agreement to manufacture certain quantities of our marketed product... -
Page 125
...-for-sale investments by type of security were as follows (in millions): Gross unrealized gains Gross unrealized losses Type of security as of December 31, 2012 Amortized cost Estimated fair value U.S. Treasury securities Other government-related debt securities: U.S. Foreign and other Corporate... -
Page 126
... and acceptable levels of risk. Our investment policy limits interest-bearing security investments to certain types of debt and money market instruments issued by institutions with primarily investment grade credit ratings and places restrictions on maturities and concentration by asset class and... -
Page 127
...December 31, 2012 and 2011, we believe the cost bases for our available-for-sale investments were recoverable in all material respects. 10. Inventories Inventories consisted of the following as of December 31, 2012 and 2011 (in millions): 2012 2011 Raw materials Work in process Finished goods Total... -
Page 128
... in Cost of sales (excludes amortization of certain acquired intangible assets) and Selling, general and administrative expense in the Consolidated Statements of Income. During the years ended December 31, 2012, 2011 and 2010, we recognized amortization charges associated with our finite-lived... -
Page 129
... liabilities consisted of the following as of December 31, 2012 and 2011 (in millions): 2012 2011 Sales deductions Employee compensation and benefits Sales returns reserve Legal reserve Other Total accrued liabilities 14. Financing arrangements $ $ 1,129 1,010 346 - 2,306 4,791 $ $ 1,326 916... -
Page 130
... this conversion rate was adjusted for certain transactions with respect to our common stock, including payment of cash dividends. Prior to their maturity, the 0.375% 2013 Convertible Notes could only be converted: (i) during any calendar quarter if the closing price of our common stock exceeded 130... -
Page 131
... our option in cash or shares of our common stock, and these contracts met all of the applicable criteria for equity classification under the applicable accounting standards, the cost of the convertible note hedges and net proceeds from the sale of the warrants are classified in Stockholders' equity... -
Page 132
... this facility. In connection with the new revolving credit agreement we terminated our prior $2.3 billion revolving credit agreement that was scheduled to expire in November 2012. In March 2011, we filed a shelf registration statement with the U.S. Securities and Exchange Commission to replace an... -
Page 133
...cash dividends of $0.36 per share of common stock, which were paid on March 7, June 7, September 7 and December 7, 2012, respectively. Additionally, on December 13, 2012, the Board of Directors declared a quarterly cash dividend of $0.47 per share of common stock, which will be paid on March 7, 2013... -
Page 134
... based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company's assumptions about the inputs that market participants would use in pricing the asset or liability and are developed based on the best information available in the... -
Page 135
...): Quoted prices in active markets for identical assets (Level 1) Significant other observable inputs (Level 2) Significant unobservable inputs (Level 3) Fair value measurement as of December 31, 2012, using: Total Assets: Available-for-sale investments: U.S. Treasury securities Other government... -
Page 136
...measurement as of December 31, 2011, using: Quoted prices in active markets for identical assets (Level 1) Significant other observable inputs (Level 2) Significant unobservable inputs (Level 3) Total Assets: Available-for-sale investments: U.S. Treasury securities Other government-related debt... -
Page 137
... Potential payments are also due upon the first commercial sale in each of the United States and the EU following receipt of marketing approval which includes use of the product in specified patient populations and upon achievement of specified levels of sales within specified periods of time. These... -
Page 138
... for speculative trading purposes. Cash flow hedges We are exposed to possible changes in the values of certain anticipated foreign currency cash flows resulting from changes in foreign currency exchange rates, associated primarily with our euro-denominated international product sales. Increases and... -
Page 139
...Income location 2012 2011 2010 Foreign currency contracts Cross-currency swap contracts Forward interest rate contracts Total Product sales ...effectively converted a fixed interest rate coupon to a floating LIBOR-based coupon over the lives of the respective notes. While outstanding, the rates... -
Page 140
... location 2012 2011...generally defined as having either a credit rating that is below investment grade or a materially weaker creditworthiness after the change in control. If these events were to occur, the counterparties would have the right, but not the obligation, to close the contracts under early... -
Page 141
...plaintiff to a class action with thousands of putative class members. These legal proceedings, as well as other matters, involve various aspects of our business and a variety of claims (including but not limited to patent infringement, marketing, pricing and trade practices and securities law), some... -
Page 142
..., among other things, in connection with wrongful termination lawsuits and potential violations of the Health Insurance Portability and Accountability Act. On February 25, 2009, the case was reassigned to a judge in the Complex Department of the Los Angeles Superior Court. Amgen and the individual... -
Page 143
...® clinical studies, marketed both Aranesp® and EPOGEN® for off-label uses and that these actions or inactions as well as the Amgen market strategy caused damage to the Company resulting in several inquiries, investigations and lawsuits that are costly to defend. The complaint also alleges insider... -
Page 144
... from the Attorney General of the State of New York seeking documents related to Amgen's promotional activities, sales and marketing activities, medical education, clinical studies, pricing and contracting, license and distribution agreements and corporate communications. Amgen fully cooperated in... -
Page 145
... on December 19, 2012. In connection with entering into the Settlement Agreement, Amgen also entered into a corporate integrity agreement with the Office of Inspector General of the U.S. Department of Health and Human Services that requires Amgen to maintain its corporate compliance program and to... -
Page 146
... that Amgen's promotional, contracting, sales and marketing activities and arrangements relating to Aranesp®, NEUPOGEN®, Neulasta®, XGEVA®, Prolia®, Vectibix® and Nplate® caused the submission of various false claims under the Federal Civil False Claims Act and various State False Claims Acts... -
Page 147
...®/Mimpara® Vectibix® Nplate® XGEVA® Prolia® Other Total product sales Other revenues Total revenues Geographic information Outside the United States, we sell products principally in Europe and Canada. The geographic classification of product sales was based on the location of the customer... -
Page 148
... net trade receivables on a combined basis. At December 31, 2012 and 2011, 36% and 39%, respectively, of trade receivables, net were due from customers located outside the United States, primarily in Europe. Our total allowance for doubtful accounts as of December 31, 2012 and 2011, was not material... -
Page 149
...31 $ $ $ $ $ $ $ $ 2011 Quarters ended September 30 Product sales Gross profit from product sales Net income Earnings per share: Basic Diluted (1)... settlement charge ($705 million, net of tax) in connection with an agreement in principle to settle allegations related to our sales and marketing... -
Page 150
SCHEDULE II AMGEN INC. VALUATION AND QUALIFYING ACCOUNTS Years ended December 31, 2012, 2011 and 2010 (In millions) Additions charged to costs and expenses Balance at end of period Allowance for doubtful accounts Balance at beginning of period Other additions Deductions Year ended December 31, ...