Pfizer 2007 Annual Report Download
Download and view the complete annual report
Please find the complete 2007 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Pfizer Inc.
2007 Financial Report
Table of contents
-
Page 1
Pfizer Inc. 2007 Financial Report -
Page 2
... Public Accounting Firm on Internal Control Over Financial Reporting ...Consolidated Statements of Income ...Consolidated Balance Sheets ...Consolidated Statements of Shareholders' Equity ...Consolidated Statements of Cash Flows ...Notes to Consolidated Financial Statements ...Quarterly Consolidated... -
Page 3
... in healthcare systems and their related costs. Our revenues are derived from the sale of our products, as well as through alliance agreements, under which we co-promote products discovered by other companies. Our Pharmaceutical segment represented approximately 92% of our total revenues in... -
Page 4
...). The asset write-offs (intangibles, inventory and ï¬xed assets) represent non-cash charges. The other exit costs, primarily severance, contract and other termination costs, as well as other liabilities, are associated with marketing and research programs, and manufacturing operations related to... -
Page 5
...Looking Information and Factors That May Affect Future Results" section of this Financial Review. Such industry-wide factors include pricing and access, intellectual property rights, product competition, the regulatory environment, pipeline productivity and the changing business environment. Pricing... -
Page 6
... the development process to ensure that our approved products will deliver the value expected by those payers. We will continue to be a constructive force in helping to shape healthcare policy and regulation of our products. • • • • • Intellectual Property Rights Our business model is... -
Page 7
... at our business model and examining it from all angles. We believe there are opportunities to better manage our products' growth and development throughout their entire time on the market and bring innovation to our "go to market" promotional and commercial strategies. We plan to develop ways to... -
Page 8
... at our business model and examining it from all angles. We believe there are opportunities to better manage our products' growth and development throughout their entire time on the market and bring innovation to our "go to market" promotional and commercial strategies. We plan to develop ways to... -
Page 9
... demand for high-quality healthcare and offer the best potential for our products. Worldwide emphasis on the need to ï¬nd solutions to difï¬cult problems in healthcare systems. • • • in-need, in January 2007, PGRD announced a number of actions to transform the research division. Many of... -
Page 10
..., co-promotion agreements and acquisitions. Our business development strategy targets a number of growth opportunities, including biologics, oncology, diabetes, Alzheimer's disease, cardiovascular disease, vaccines and other products and services that seek to provide valuable healthcare solutions... -
Page 11
...manufacture and sell Exubera, an inhaled form of insulin, and the insulin-production business and facilities located in Frankfurt, Germany, previously jointly owned by Pï¬zer and sanoï¬-aventis, for approximately $1.4 billion in cash (including transaction costs). Substantially all assets recorded... -
Page 12
... to the new owner: Revenues of $219 million; Cost of sales of $194 million; Selling, informational and administrative expenses of $15 million; and Other (income)/deductions-net of $16 million in income. • In February 2008, we signed an agreement to acquire all issued and outstanding shares of... -
Page 13
...this Financial Review. Acquisitions Our consolidated ï¬nancial statements and results of operations reï¬,ect an acquired business after the completion of the acquisition and are not restated. We account for acquired businesses using the purchase method of accounting, which requires that the assets... -
Page 14
... discovered by other companies. Alliance revenues are earned when our co-promotion partners ship the related product and title passes to their customer. These revenues are primarily based upon a percentage of our co-promotion partners' net sales. Expenses for selling and marketing these products... -
Page 15
...required rate of return used in the discounted cash ï¬,ow method, which reï¬,ects capital market conditions and the speciï¬c risks associated with the business segment. Other estimates inherent in the "income approach" include long-term growth rates and cash ï¬,ow forecasts for the business segment... -
Page 16
Financial Review Pï¬zer Inc and Subsidiary Companies include discount rate; expected salary increases; certain employeerelated factors, such as turnover, retirement age and mortality (life expectancy); expected return on assets; and healthcare cost trend rates. Our assumptions reï¬,ect our ... -
Page 17
... Health -The Animal Health segment includes products that prevent and treat diseases in livestock and companion animals. Total Revenues by Business Segment 5.4% 2.8% 4.8% 2.0% 4.6% 2.0% 2007 2006 2005 91.8% 93.2% 93.4% PHARMACEUTICAL ANIMAL HEALTH CORPORATE/OTHER 2007 Financial Report... -
Page 18
... area follow: YEAR ENDED DEC. 31, _____ WORLDWIDE U.S. INTERNATIONAL (MILLIONS OF DOLLARS) % CHANGE _____ WORLDWIDE U.S. INTERNATIONAL 07/06 06/05 07/06 06/05 07/06 06/05 2007 2006 2005 2007 2006 2005 2007 2006 2005 Revenues: Pharmaceutical Animal Health Corporate/Other Total Revenues... -
Page 19
Financial Review Pï¬zer Inc and Subsidiary Companies Revenues-Major Pharmaceutical Products Revenue information for several of our major Pharmaceutical products follow: (MILLIONS OF DOLLARS) PRODUCT PRIMARY INDICATIONS YEAR ENDED DEC. 31, % CHANGE 2007 2006 2005 07/06 06/05 Cardiovascular and... -
Page 20
... state-funded National Health Service in the U.K., following a positive appraisal decision in May 2007. Our strategy for this innovative medicine is to build a sustainable, medically supported market over time and to seek to secure reimbursement-initiatives that we believe will drive future growth... -
Page 21
... to multiple anti-retroviral agents. A diagnostic test conï¬rms whether a patient is infected with CCR5tropic HIV-1, which is also known as "R5-virus." Viagra remains the leading treatment for erectile dysfunction and one of the world's most recognized pharmaceutical brands. Viagra revenues grew... -
Page 22
... the sale of our Consumer Healthcare business, we ceased selling this product in late January 2008. Alliance revenues reï¬,ect revenues primarily associated with our co-promotion of Aricept, Rebif and Spiriva. -Aricept, discovered and developed by our alliance partner Eisai Co., Ltd, is the world... -
Page 23
...We are working with Schwarz Pharma, the licensor, to scale up manufacturing and identify manufacturing site alternatives. Launch is planned for mid-2008 in Europe and, ...or planned clinical trials for additional uses and dosage forms for our in-line products include: PRODUCT INDICATION Celebrex Acute... -
Page 24
... million in write-offs of inventory and exit costs in 2005 related to suspension of sales and marketing of Bextra. • Selling, Informational and Administrative (SI&A) Expenses SI&A expenses in 2007 were comparable to 2006, which reï¬,ects: The increase in Animal Health revenues in 2006, compared... -
Page 25
... products; and higher payments for intellectual property rights, discussed below, among other factors, partially offset by: • • a one-time R&D milestone due to us from sanofi-aventis (approximately $118 million); and savings related to our cost-reduction initiatives. 2007 Financial Report... -
Page 26
... and the restructuring of our worldwide marketing and research and development operations, and the implementation costs primarily relate to accelerated depreciation of certain assets, as well as system and process standardization and the expansion of shared services. The components of restructuring... -
Page 27
... analysis of our operating results that is prepared on an Adjusted income basis; Our annual budgets are prepared on an Adjusted income basis; and Annual and long-term compensation, including annual cash bonuses, merit-based salary adjustments and share-based payments for various levels of management... -
Page 28
Financial Review Pï¬zer Inc and Subsidiary Companies intangible assets for the increase to fair value. Therefore, the Adjusted income measure includes the revenues earned upon the sale of the acquired products without considering the aforementioned signiï¬cant charges. Certain of the purchase-... -
Page 29
... income follows: YEAR ENDED DEC. 31, _____ 2007 2006 2005 % CHANGE _____ 07/06 06/05 (MILLIONS OF DOLLARS) Reported net income $ 8,144 $19,337 $ 8,085 Purchase accounting adjustments- net of tax 2,511 3,131 3,967 Acquisition-related costs-net of tax 10 14 599 Discontinued operations- net of... -
Page 30
... primarily related to our Consumer Healthcare business. (See Notes to Consolidated Financial Statements-Note 3. Discontinued Operations.) Included in Cost of sales ($700 million), Selling, informational and administrative expenses ($334 million), Research and development expenses ($416 million) and... -
Page 31
...16.6 billion from the sale of our Consumer Healthcare business on December 20, 2006. We rely largely on operating cash ï¬,ow, short-term investments, long-term debt and short-term commercial paper borrowings to provide for the working capital needs of our operations, including our R&D activities. We... -
Page 32
... Cash and cash equivalents and short-term investments and loans Working capital(a) Ratio of current assets to current liabilities Shareholders' equity per common share(b) (a) $26,092 $25,014 2.15:1 $ 9.65 $28,227 $25,559 2.16:1 $ 10.05 (b) Includes $16.6 billion related to developed technology... -
Page 33
... 2008, we announced a new $5 billion share-purchase program, which will be funded by operating cash ï¬,ows. A summary of common stock purchases follows: SHARES OF COMMON STOCK PURCHASED AVERAGE PER-SHARE PRICE PAID TOTAL COST OF COMMON STOCK PURCHASED Our net cash provided by investing activities... -
Page 34
... our businesses. Our dividends are funded from operating cash ï¬,ows, our ï¬nancial asset portfolio and short-term commercial paper borrowings and are not restricted by debt covenants. To the extent we have additional capital in excess of investment opportunities, we typically offer a return to... -
Page 35
... competitor products; Impact of existing and future legislation and regulatory provisions on product exclusivity; Trends toward managed care and healthcare cost containment; U.S. legislation or regulatory action affecting, among other things, pharmaceutical product pricing, reimbursement or access... -
Page 36
.... We are also subject to interest rate risk on euro debt, investments and currency swaps, Swedish krona currency swaps, and on Japanese yen short and long-term borrowings and currency swaps. We invest, loan and borrow primarily on a short-term or variable-rate basis. From 34 2007 Financial Report -
Page 37
Financial Review Pï¬zer Inc and Subsidiary Companies time to time, depending on market conditions, we will ï¬x interest rates either through entering into ï¬xed-rate investments and borrowings or through the use of derivative ï¬nancial instruments such as interest rate swaps. Our ï¬nancial ... -
Page 38
... policies applied by the Company in its financial statements, as well as alternative treatments. Management represented to the Committee that the Company's consolidated ï¬nancial statements were prepared in accordance with accounting principles generally accepted in the United States of America... -
Page 39
...), and our report dated February 29, 2008 expressed an unqualiï¬ed opinion on the effective operation of the Company's internal control over ï¬nancial reporting. As discussed in the Notes to the Consolidated Financial Statements-Note 1D. Significant Accounting Policies: New Accounting Standards... -
Page 40
... States), the consolidated balance sheets of Pï¬zer Inc and Subsidiary Companies as of December 31, 2007 and 2006, and the related consolidated statements of income, shareholders' equity, and cash ï¬,ows for each of the years in the three-year period ended December 31, 2007, and our report dated... -
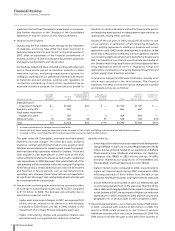
Page 41
...Companies (MILLIONS, EXCEPT PER COMMON SHARE DATA) YEAR ENDED DECEMBER 31, _____ 2007 2006 2005 Revenues Costs and expenses: Cost of sales(a) Selling, informational and administrative expenses(a) Research and development expenses(a) Amortization of intangible assets Acquisition-related in-process... -
Page 42
... COMMON SHARE DATA) AS OF DECEMBER 31, _____ 2007 2006 Assets Cash and cash equivalents Short-term investments Accounts receivable, less allowance for doubtful accounts: 2007-$223; 2006-$204 Short-term loans Inventories Prepaid expenses and taxes Assets held for sale Total current assets Long-term... -
Page 43
Consolidated Statements of Shareholders' Equity Pï¬zer Inc and Subsidiary Companies PREFERRED STOCK COMMON STOCK (MILLIONS, EXCEPT PREFERRED SHARES) SHARES STATED VALUE ADDITIONAL PAID-IN SHARES PAR VALUE CAPITAL EMPLOYEE BENEFIT TRUST TREASURY STOCK SHARES FAIR VALUE SHARES COST RETAINED ... -
Page 44
...businesses acquired and divested: Accounts receivable Inventories Prepaid and other assets Accounts payable and accrued liabilities Income taxes payable Other liabilities Net cash provided by operating activities Investing Activities Purchases of property, plant and equipment Purchases of short-term... -
Page 45
... Policies A. Consolidation and Basis of Presentation The consolidated ï¬nancial statements include our parent company and all subsidiaries, including those operating outside the U.S., and are prepared in accordance with accounting principles generally accepted in the United States of America... -
Page 46
... historical rates. G. Revenues Revenue Recognition-We record revenues from product sales when the goods are shipped and title passes to the customer. At the time of sale, we also record estimates for a variety of sales deductions, such as sales rebates, discounts and incentives, and product returns... -
Page 47
... to their customer. Alliance revenues are primarily based upon a percentage of our co-promotion partners' net sales. Expenses for selling and marketing these products are included in Selling, informational and administrative expenses. agreement term or the expected product life cycle, whichever... -
Page 48
... basis over the vesting terms into Cost of sales, Selling, informational and administrative expenses and Research and development expenses, as appropriate. In 2005 and earlier years, grants under stock option and performancecontingent share award programs were accounted for using the intrinsic value... -
Page 49
...circumstances of the service, location and/or business need. The agreements can include the following: manufacturing and product supply, logistics, customer service, support of financial processes, procurement, human resources, facilities management, data collection and information services. Most of... -
Page 50
... the restructuring of our worldwide sales, marketing and research and development operations, while the implementation costs primarily relate to accelerated depreciation of certain assets, as well as system and process standardization and the expansion of shared services. 48 2007 Financial Report -
Page 51
... have been formally terminated. Employee termination costs are recorded when the actions are probable and estimable and include accrued severance beneï¬ts, pension and postretirement beneï¬ts. Asset impairments primarily include charges to write down property, plant and equipment. Other primarily... -
Page 52
... accounting principles follows: YEAR ENDED DEC. 31, _____ 2007 2006 2005 (MILLIONS OF DOLLARS) United States: Taxes currently payable: Federal State and local Deferred income taxes Total U.S. tax (beneï¬t)/provision International: Taxes currently payable Deferred income taxes Total international... -
Page 53
...-time audit process. All other tax years in the U.S. for Pï¬zer Inc. are closed under the statute of limitations. With respect to Pharmacia Corporation, the IRS is currently conducting an audit for the year 2003 through the date of merger with Pï¬zer (April 16, 2003). In addition to the open audit... -
Page 54
... common to multinational corporations or the expiration of the statute of limitations in multiple jurisdictions. Tax liabilities associated with uncertain tax positions represent unrecognized tax benefits, which arise when the estimated benefit recorded in our financial statements differs from the... -
Page 55
... ADJUSTMENT AND OTHER DERIVATIVE FINANCIAL INSTRUMENTS AVAILABLEFOR-SALE SECURITIES BENEFIT PLANS PRIOR SERVICE (COSTS)/CREDITS AND OTHER MINIMUM PENSION LIABILITY ACCUMULATED OTHER COMPREHENSIVE INCOME/ (EXPENSE) (MILLIONS OF DOLLARS) ACTUARIAL GAINS/(LOSSES) Balance, January 1, 2005 $ 2,594... -
Page 56
...the consolidated balance sheets as of December 31: (MILLIONS OF DOLLARS) 2007 2006 2007 2006 Trading investments(a) Amortized cost and fair value of available-for-sale debt securities:(b) Western European and other government debt Corporate debt Western European and other government agency debt... -
Page 57
... rates. We seek to manage our foreign exchange risk in part through operational means, including managing expected same currency revenues in relation to same currency costs and same currency assets in relation to same currency liabilities. Depending on market conditions, foreign exchange risk... -
Page 58
... with long-term debt obligations to ï¬,oating rates (see also Note 10C. Financial Instruments: Long-Term Debt). All derivative contracts used to manage interest rate risk are measured at fair value and reported as assets or liabilities on the balance sheet. Changes in fair value are reported in... -
Page 59
...Intangible Assets A. Goodwill The changes in the carrying amount of goodwill by segment for the years ended December 31, 2007 and 2006, follow: (MILLIONS OF DOLLARS) F. Credit Risk On an ongoing basis, we review the creditworthiness of counterparties to foreign exchange and interest rate agreements... -
Page 60
... Subsidiary Companies Developed technology rights represent the amortized value associated with developed technology, which has been acquired from third parties and which can include the right to develop, use, market, sell and/or offer for sale the product, compounds and intellectual property that... -
Page 61
... into 2008 net periodic beneï¬t costs: PENSION PLANS U.S. SUPPLEMENTAL (NON-QUALIFIED) POSTRETIREMENT PLANS (MILLIONS OF DOLLARS) U.S. QUALIFIED INTERNATIONAL Actuarial losses Prior service costs/(credits) and other Total $34 3 $37 $35 (3) $32 $44 1 $45 $25 2 $27 2007 Financial Report 59 -
Page 62
... are reviewed on an annual basis. We revise these assumptions based on an annual evaluation of long-term trends, as well as market conditions, that may have an impact on the cost of providing retirement beneï¬ts. The expected rates of return on plan assets for our U.S. qualiï¬ed, international and... -
Page 63
Notes to Consolidated Financial Statements Pï¬zer Inc and Subsidiary Companies D. Obligations and Funded Status The following table presents an analysis of the changes in 2007 and 2006 in the beneï¬t obligations, the plan assets and the funded status of our U.S. qualiï¬ed, U.S. supplemental (non... -
Page 64
...consolidated balance sheet as of December 31 follow: PENSION PLANS U.S. SUPPLEMENTAL (NON-QUALIFIED) 2007 2006 POSTRETIREMENT PLANS 2007 2006 (MILLIONS OF DOLLARS) U.S. QUALIFIED 2007 2006 INTERNATIONAL 2007 2006 Noncurrent assets(a) Current liabilities(b) Noncurrent liabilities(c) Funded status... -
Page 65
... is held in an employee stock ownership plan. We recorded charges related to our plans of $203 million in 2007, $222 million in 2006 and $234 million in 2005. All long-term asset allocation targets reï¬,ect our asset class return expectations and tolerance for investment risk within the context of... -
Page 66
... our employee beneï¬t plans through the use of its holdings of Pï¬zer Inc stock. The consolidated balance sheets reï¬,ect the fair value of the shares owned by the EBT as a reduction of Shareholders' equity. SHARES OF AVERAGE TOTAL COST OF COMMON STOCK PER-SHARE COMMON STOCK PURCHASED PRICE PAID... -
Page 67
... amortized on an even basis over the vesting term into Cost of sales, Selling, informational and administrative expenses and Research and development expenses, as appropriate. In 2005 and earlier years, stock options were accounted for under APB No. 25, using the intrinsic value method in the income... -
Page 68
...in virtually all instances, the units vest after three years of continuous service from the grant date and the fair values are amortized on an even basis over the vesting term into Cost of sales, Selling, informational and administrative expenses and Research and development expenses, as appropriate... -
Page 69
... an even basis over the vesting term into Cost of sales, Selling, informational and administrative expenses and Research and development expenses, as appropriate. For grants in 2005 and earlier years, PCSA grants are accounted for using the intrinsic value method in the income statement. Senior and... -
Page 70
... the end of a vesting term, a specified number of shares of our common stock, and which also entitle the holder to receive dividends paid on such grants, are accounted for at fair value at the date of grant. Senior and key members of management received restricted stock awards prior to 2005. In most... -
Page 71
... operating leases as of December 31 for the following years: (MILLIONS OF DOLLARS) 2008 2009 2010 2011 2012 AFTER 2012 Lease commitments $212 $192 $151 $99 $76 $788 19. Insurance Our insurance coverage reflects market conditions (including cost and availability) existing at the time... -
Page 72
... generic manufacturers who are seeking to market their own amlodipine products in Canada. In February 2008, a trial was held in the Federal Court of Canada in Toronto in our challenge against Cobalt, and we are awaiting the decision. Our Norvasc patent in Canada expires in August 2010. Celebrex... -
Page 73
..., Louisiana Health Service Indemnity Company and Eastern States Health and Welfare Fund ï¬led a consolidated complaint against Warner-Lambert in the U.S. District Court for the Southern District of New York purportedly on behalf of a class consisting of all health beneï¬t providers that paid for... -
Page 74
... the gastrointestinal effects of Celebrex. These cases were consolidated for pre-trial proceedings in the District of New Jersey (Alaska Electrical Pension Fund et al. v. Pharmacia Corporation et al.). In January 2007, the court certiï¬ed a class consisting of all persons who purchased Pharmacia... -
Page 75
... to misrepresent the safety of Celebrex and, in certain of the cases, Bextra; and (iii) purported class actions filed by persons who claim to be participants in the Pï¬zer or Pharmacia Savings Plan alleging that Pfizer and certain current and former officers, directors and employees of Pï¬zer or... -
Page 76
... the Company engaged in false and misleading advertising in violation of state consumer protection laws by allegedly promoting Lipitor for the prevention of heart disease in women (regardless of age) and men over age 55 who in each case had no history of heart disease or diabetes. The action sought... -
Page 77
...and Other Matters Average Wholesale Price Litigation A number of states as well as most counties in New York have sued Pharmacia, Pfizer and other pharmaceutical manufacturers alleging that they provided average wholesale price (AWP) information for certain of their products that was higher than the... -
Page 78
...Pension Plan In 2006, several current and former employees of Pharmacia Corporation filed a purported class action in the U.S. District Court for the Southern District of Illinois against the Pharmacia Cash Balance Pension Plan (the Plan), Pharmacia Corporation, Pharmacia & Upjohn Company and Pfizer... -
Page 79
... asset impairments and costs related to our cost-reduction initiatives. Each segment is managed separately and offers different products requiring different marketing and distribution strategies. We sell our products primarily to customers in the wholesale sector. In 2007, sales to our three largest... -
Page 80
... Financial Statements Pï¬zer Inc and Subsidiary Companies The following tables present segment, geographic and revenue information: Segment FOR/AS OF THE YEAR ENDED DEC. 31, (MILLIONS OF DOLLARS) 2007 2006 2005 Revenues Pharmaceutical Animal Health Corporate/Other(a) Total revenues Segment... -
Page 81
...and Subsidiary Companies Geographic FOR/AS OF THE YEAR ENDED DEC. 31, (MILLIONS OF DOLLARS) 2007 2006 2005 Revenues United States(a) Europe/Canada(b) Japan/Asia(c) Latin America/AFME(d) Consolidated Long-lived assets United States(a) Europe/Canada(b) Japan/Asia(c) Latin America/AFME(d) (e) $23... -
Page 82
... Cash dividends paid per common share Stock prices High Low Basic and diluted EPS are computed independently for each of the periods presented. Accordingly, the sum of the quarterly EPS amounts may not agree to the total for the year. Acquisition-related in-process research and development charges... -
Page 83
... share Stock prices High Low Basic and diluted EPS are computed independently for each of the periods presented. Accordingly, the sum of the quarterly EPS amounts may not agree to the total for the year. All ï¬nancial information reï¬,ects our Consumer Healthcare business as discontinued operations... -
Page 84
... accounting principles-net of tax(d) Net income Effective tax rate-continuing operations Depreciation and amortization(e) Property, plant and equipment additions(e) Cash dividends paid Working capital(f) Property, plant and equipment, less accumulated depreciation Total assets(f) Long-term debt Long... -
Page 85
... Subsidiary Companies On April 16, 2003, Pï¬zer acquired Pharmacia Corporation in a transaction accounted for as a purchase. All ï¬nancial information reï¬,ects the following as discontinued operations: our Consumer Healthcare, in-vitro allergy and autoimmune diagnostic testing, certain European...