Pfizer 2015 Annual Report Download - page 50
Download and view the complete annual report
Please find page 50 of the 2015 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
2015 Financial Report
49
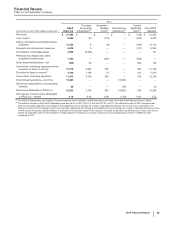
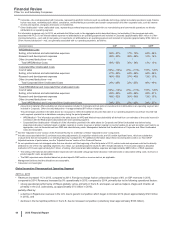
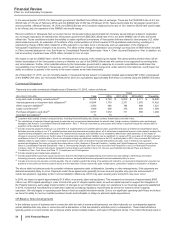
ANALYSIS OF THE CONSOLIDATED BALANCE SHEETS
For information about certain of our financial assets and liabilities, including Cash and cash equivalents, Short-term investments, Long-term
investments, Short-term borrowings, including current portion of long-term debt, and Long-term debt, see “Analysis of the Consolidated
Statements of Cash Flows” section of this Financial Review, the “Analysis of Financial Condition, Liquidity and Capital Resources: Selected
Measures of Liquidity and Capital Resources” section of this Financial Review and Notes to Consolidated Financial Statements—Note 7.
Financial Instruments.
For information about certain balances in Trade accounts receivable, less allowance for doubtful accounts, see also the “Analysis of Financial
Condition, Liquidity and Capital Resources: Selected Measures of Liquidity and Capital Resources: Accounts Receivable” section of this
Financial Review.
For information about events and circumstances impacting our tax-related accounts, see Notes to Consolidated Financial Statements—Note
5. Tax Matters.
For a description of changes in Total Equity, see the consolidated statements of equity.
The changes in our asset and liability accounts as of December 31, 2015, compared to December 31, 2014, generally reflect, among other
things, the impact of assets acquired and liabilities assumed as part of the acquisition of Hospira (see Notes to Consolidated Financial
Statements––Note 2A. Acquisitions, Licensing Agreements, Collaborative Arrangements, Divestitures, Equity-Method Investments and Cost-
Method Investment: Acquisitions), and decreases due to changes in foreign currency exchange rates, some of which impacts were significant.
The following explanations exclude the impact of the acquisition of Hospira and foreign exchange:
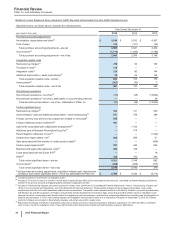
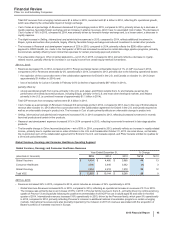
• For Trade accounts receivable, less allowance for doubtful accounts, the change also reflects the timing of sales and collections in the
normal course of business.
• For Inventories, the change also reflects an increase to inventory, resulting from a change in the profit deferred in inventory relating to
inventory that had not been sold to third parties, inventory acquired as part of the acquisition of Baxter’s portfolio of marketed vaccines,
recorded at acquisition-date fair value as well as inventory builds in the normal course of business, partially offset by planned inventory
reductions.
• For Other current assets, the change also reflects the decrease in the receivables associated with our derivative financial instruments as
well as the timing of receipts and payments in the normal course of business.
• For Property, plant and equipment, less accumulated depreciation, the change also reflects depreciation, mainly offset by capital additions.
• For Identifiable intangible assets, less accumulated amortization, the change also reflects amortization and to a lesser extent impairments,
partially offset by identifiable intangible assets acquired as part of the acquisition of Baxter’s portfolio of marketed vaccines. For additional
information about our intangible assets, see Notes to Consolidated Financial Statements—Note 10A. Identifiable Intangible Assets and
Goodwill: Identifiable Intangible Assets. For additional information about the asset impairment charges, see Notes to Consolidated
Financial Statements—Note 4. Other (Income)/Deductions—Net. For additional information about the assets acquired as part of the
acquisition of Baxter’s portfolio of marketed vaccines, see Notes to Consolidated Financial Statements—Note 2A. Acquisitions, Licensing
Agreements, Collaborative Arrangements, Divestitures, Equity-Method Investments and Cost-Method Investment: Acquisitions.
• For Trade accounts payable, the change also reflects the timing of purchases and payments in the normal course of business.
• For Accrued compensation and related items, the change also reflects a higher bonus accrual attributable to performance and a change in
the structure of our compensation whereby fixed compensation for certain previously non-bonus eligible colleagues was reduced and
replaced with an equal amount of variable compensation tied to the performance of the Company and is paid annually.
• For Other current liabilities, the change also reflects an increase in the payables associated with our derivative financial instruments, a net
increase in legal-related liabilities, mainly the accrual for the agreement in principle to resolve claims relating to Protonix, partially offset by
payments of certain legal claims, as well as the timing of other payments and accruals in the normal course of business. For additional
information, see Notes to Consolidated Financial Statements—Note 17A4. Commitments and Contingencies: Legal Proceedings—
Government Investigations.
• For Pension benefit obligations, net, and Postretirement benefit obligations, net, the change reflects, among other things, a $1.0 billion
voluntary pension contribution in January 2015, an increase in our discount rate assumptions used in the measurement of the plan
obligations, a $507 million reduction in our U.S. Postretirement Plan obligation due to a plan amendment approved in June 2015 that
introduced a cap on costs for certain groups within the plan, and a rise in the comparative strength of the U.S. dollar, as compared to other
currencies. For additional information, see Notes to Consolidated Financial Statements—Note 11. Pension and Postretirement Benefit
Plans and Defined Contribution Plans.
• For Other noncurrent liabilities, the change reflects an increase in the payables associated with our derivative financial instruments and, to
a lesser extent, the deferral of an upfront payment received as part of our tanezumab collaborative arrangement, partially offset by other
payments and changes in accruals in the normal course of business.
• For Accumulated other comprehensive loss, the change primarily reflects foreign currency translation adjustments for 2015. For additional
information see the “Analysis of the Consolidated Statements of Comprehensive Income” section of this Financial Review.