Pfizer 2015 Annual Report Download - page 31
Download and view the complete annual report
Please find page 31 of the 2015 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
30
2015 Financial Report
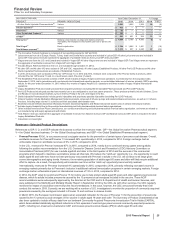
NEW DRUG CANDIDATES IN LATE-STAGE DEVELOPMENT
CANDIDATE PROPOSED INDICATION
Avelumab (PF-06834635)
(MSB0010718C)
A monoclonal antibody that inhibits PD-L1 for the first-line treatment of stage IIIb/IV non-small cell lung cancer,
which is being developed in collaboration with Merck KGaA, Germany
Avelumab (PF-06834635)
(MSB0010718C)
A monoclonal antibody that inhibits PD-L1 for treatment of stage IIIb/IV non-small cell lung cancer that has
progressed after a platinum-containing doublet, which is being developed in collaboration with Merck KGaA,
Germany
Avelumab (PF-06834635)
(MSB0010718C)
A monoclonal antibody that inhibits PD-L1 for treatment of platinum-resistant/refractory ovarian cancer, which is
being developed in collaboration with Merck KGaA, Germany
Avelumab (PF-06834635)
(MSB0010718C)
A monoclonal antibody that inhibits PD-L1 for maintenance treatment, in the first-line setting, for patients with
urothelial cancer, which is being developed in collaboration with Merck KGaA, Germany
Avelumab (PF-06834635)
(MSB0010718C)
A monoclonal antibody that inhibits PD-L1 for maintenance treatment of advanced or metastatic gastric/gastro-
esophageal junction cancers, which is being developed in collaboration with Merck KGaA, Germany
Avelumab (PF-06834635)
(MSB0010718C)
Third-line treatment in advanced or metastatic gastric/gastro-esophageal junction cancers, which is being
developed in collaboration with Merck KGaA, Germany
Bococizumab A monoclonal antibody that inhibits PCSK9 for the treatment of hyperlipidemia and prevention of cardiovascular
events
Dacomitinib A pan-HER tyrosine kinase inhibitor for the first-line treatment of patients with advanced non-small cell lung
cancer with EGFR activating mutations, which is being developed in collaboration with SFJ Pharmaceuticals
Group
Ertugliflozin An oral SGLT2 inhibitor for the treatment of patients with type 2 diabetes, which is being developed in
collaboration with Merck & Co., Inc.
Inotuzumab ozogamicin An antibody drug conjugate, consisting of an anti-CD22 monotherapy antibody linked to a cytotoxic agent,
calicheamycin, for the treatment of acute lymphoblastic leukemia
PF-06836922 A long-acting hGH-CTP for the treatment of growth hormone deficiency in adults, which is being developed in
collaboration with OPKO Health, Inc.
PF-06438179(a) A potential biosimilar to Remicade® (infliximab)
PF-05280014(b) A potential biosimilar to Herceptin® (trastuzumab)
PF-05280586(c) A potential biosimilar to Rituxan® (rituximab)
PF-06439535(d) A potential biosimilar to Avastin® (bevacizumab)
PF-06410293(e) A potential biosimilar to Humira® (adalimumab)
Rivipansel (GMI-1070) A pan-selectin inhibitor for the treatment of vaso-occlusive crisis in hospitalized individuals with sickle cell
disease, which was licensed from GlycoMimetics Inc.
Tanezumab An anti-nerve growth factor monoclonal antibody for the treatment of pain, which is being developed in
collaboration with Eli Lilly & Company
Trumenba A prophylactic vaccine for active immunization to prevent invasive disease caused by Neisseria meningitidis
serogroup B in individuals 10 through 25 years of age (ex-U.S.)
(a) Remicade® is a registered trademark of Janssen Biotech, Inc. In February 2016, we divested the rights for development and commercialization of
PF-06438179, a potential biosimilar to Remicade® (infliximab) in the 28 countries that form the European Economic Area (EEA) to Sandoz, which was a
condition to the European Commission’s approval of the Hospira transaction. We retain commercialization and manufacturing rights to PF-06438179 in all
countries outside of the EEA.
(b) Herceptin® is a registered trademark of Genentech, Inc.
(c) Rituxan® is a registered trademark of Biogen MA, Inc.
(d) Avastin® is a registered trademark of Genentech, Inc.
(e) Humira® is a registered trademark of AbbVie Biotechnology Ltd.
Inflectra™
In 2009, Hospira entered into an agreement to develop and market certain biosimilar molecules with Celltrion Inc. and Celltrion Healthcare,
Co., Ltd. (collectively Celltrion) including Inflectra™ (infliximab) for patients with autoimmune diseases. In Europe, Inflectra has now launched in
36 markets. Celltrion possesses the right to commercialize its infliximab product in the same European markets as Hospira. We have
exclusive commercialization rights from Celltrion to their infliximab product in the U.S., Canada and certain other territories. In August 2014,
Celltrion submitted a potential infliximab biosimilar for FDA approval in the U.S., and in February 2016, the FDA’s Arthritis Advisory Committee
provided a non-binding recommendation to the FDA for approval across all indications. In December 2014, Hospira launched Inflectra in
Canada. Inflectra has also been approved in certain markets, where Hospira will market it as Remsima™.
In September 2015, in order to eliminate certain redundancies in Pfizer’s biosimilar drug products pipeline created as a result of the acquisition
of Hospira, Pfizer opted to return to Celltrion rights that Hospira had previously acquired to potential biosimilars to Rituxan® (rituximab) and
Herceptin® (trastuzumab). In connection with the return of the acquired rights, we incurred charges of $215 million, which are included in
Restructuring charges and certain acquisition-related costs. See Notes to Consolidated Financial Statements––Note 3. Restructuring Charges
and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives for additional information.
Additional product-related programs are in various stages of discovery and development. Also, see the discussion in the “Our Business
Development Initiatives” section of this Financial Review.