Pfizer 2015 Annual Report Download - page 3
Download and view the complete annual report
Please find page 3 of the 2015 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
2
2015 Financial Report
OVERVIEW OF OUR PERFORMANCE, OPERATING ENVIRONMENT, STRATEGY AND OUTLOOK
Our Business
We apply science and our global resources to bring therapies to people that extend and significantly improve their lives through the discovery,
development and manufacture of healthcare products. Our global portfolio includes medicines, vaccines and medical devices, as well as many
of the world’s best-known consumer healthcare products. We work across developed and emerging markets to advance wellness, prevention,
treatments and cures that challenge the most feared diseases of our time. We collaborate with healthcare providers, governments and local
communities to support and expand access to reliable, affordable healthcare around the world. Our revenues are derived from the sale of our
products and, to a much lesser extent, from alliance agreements, under which we co-promote products discovered by other companies
(Alliance revenues).
The majority of our revenues come from the manufacture and sale of biopharmaceutical products. The biopharmaceutical industry is highly
competitive and highly regulated. As a result, we face a number of industry-specific factors and challenges which can significantly impact our
results. These factors include, among others: the loss or expiration of intellectual property rights and the expiration of co-promotion and
licensing rights, healthcare legislation, pipeline productivity, the regulatory environment, pricing and access pressures and competition. We
also face challenges as a result of the global economic environment. For additional information about these factors and challenges, see the
“Our Operating Environment” section of this Financial Review and in Part I, Item 1A, “Risk Factors,” of our 2015 Annual Report on Form 10-K.
The financial information included in our consolidated financial statements for our subsidiaries operating outside the United States (U.S.) is as
of and for the year ended November 30 for each year presented. Pfizer's fiscal year-end for U.S. subsidiaries is as of and for the year ended
December 31 for each year presented.
References to developed markets in this Financial Review include the U.S., Western Europe, Japan, Canada, Australia, Scandinavia, South
Korea, Finland and New Zealand; and references to emerging markets in this Financial Review include, but are not limited to, the following
markets: Asia (excluding Japan and South Korea), Latin America, Africa, Eastern Europe, Central Europe, the Middle East and Turkey.
References to operational variances in this Financial Review refer to variances excluding the impacts of foreign exchange.
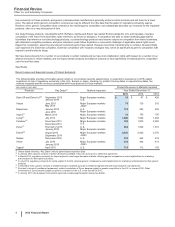
On November 23, 2015, we announced that we have entered into a definitive merger agreement with Allergan plc (Allergan), a global
pharmaceutical company incorporated in Ireland, under which we have agreed to combine with Allergan in a stock transaction valued at
$363.63 per Allergan share, for a total enterprise value of approximately $160 billion, based on the closing price of Pfizer common stock of
$32.18 on November 20, 2015 (the last trading day prior to the announcement) and certain other assumptions. Subject to the terms and
conditions of the merger agreement, the businesses of Pfizer and Allergan will be combined under a single company and Pfizer would become
a wholly-owned subsidiary of Allergan, which is organized under the laws of Ireland and which, subject to the approval by Allergan
shareholders, will be renamed “Pfizer plc”. We anticipate that the parent company will be treated as a non-U.S. corporation (and, therefore, a
non-U.S. tax resident) under the applicable U.S. federal income tax rules, although the U.S. Internal Revenue Service (IRS) may challenge
that treatment. The completion of the transaction, which is expected in the second half of 2016, is subject to certain conditions, including
receipt of regulatory approval in certain jurisdictions, including the U.S. and European Union (EU), the receipt of necessary approvals from
both Pfizer and Allergan shareholders, and the completion of Allergan’s pending divestiture of its generics business to Teva Pharmaceuticals
Industries Ltd. Readers are encouraged to review the joint proxy statement/prospectus we will file with the U.S. Securities and Exchange
Commission (SEC) seeking stockholder approval of the transaction. That document will include important information regarding the proposed
transaction. While we have taken actions and incurred costs associated with the pending combination that are reflected in our financial
statements, the pending combination with Allergan will not be reflected in our financial statements until consummation. See the “Our Business
Development Initiatives” section of this Financial Review and Notes to Consolidated Financial Statements––Note 19. Pending Combination
with Allergan for additional information.
On September 3, 2015 (the acquisition date), we acquired Hospira, Inc. (Hospira) for approximately $16.1 billion in cash ($15.7 billion, net of
cash acquired). Commencing from the acquisition date, our financial statements reflect the assets, liabilities, operating results and cash flows
of Hospira, and, in accordance with our domestic and international reporting periods, our consolidated financial statements for the year ended
December 31, 2015 reflect four months of legacy Hospira U.S. operations and three months of legacy Hospira international operations. See
Notes to Consolidated Financial Statements––Note 2A. Acquisitions, Licensing Agreements, Collaborative Arrangements, Divestitures, Equity-
Method Investments and Cost-Method Investment: Acquisitions and the “Significant Accounting Policies and Application of Critical Accounting
Estimates––Acquisition of Hospira” section of this Financial Review for additional information. Hospira is now a subsidiary of Pfizer and its
commercial operations are now included within the Global Established Pharmaceutical (GEP) segment. The combination of local Pfizer and
Hospira entities may be pending in various jurisdictions and integration is subject to completion of various local legal and regulatory steps. We
expect to generate $800 million of annual cost synergies by 2018 in connection with the Hospira acquisition. Based on our past experience,
the one-time costs to generate the synergies are expected to be approximately $1 billion (not including costs of $215 million in 2015
associated with the return of acquired in-process research and development (IPR&D) rights), incurred for up to a three-year period post-
acquisition. See the “Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives” section
of this Financial Review.
On June 24, 2013, we completed the full disposition of our Animal Health business, Zoetis Inc. (Zoetis), and recognized a gain of
approximately $10.3 billion, net of tax, in Gain on disposal of discontinued operations––net of tax in our consolidated statement of income for
the year ended December 31, 2013. The operating results of this business through June 24, 2013, the date of disposal, are reported as
Income from discontinued operations––net of tax in our consolidated statements of income. See Notes to Consolidated Financial
Statements––Note 2D. Acquisitions, Licensing Agreements, Collaborative Arrangements, Divestitures, Equity-Method Investments and Cost-
Method Investment: Divestitures for additional information.