Pfizer 2015 Annual Report Download - page 118
Download and view the complete annual report
Please find page 118 of the 2015 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
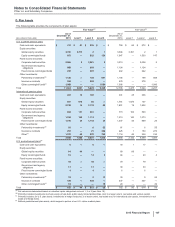
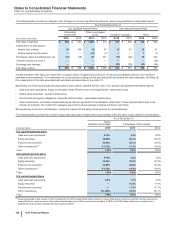
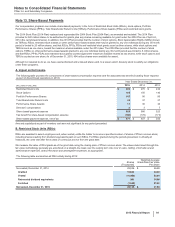
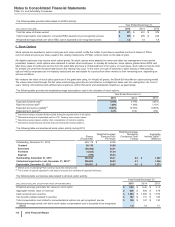
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
2015 Financial Report
117
Actions In Which We Are The Plaintiff
Sutent (sunitinib malate)
In May 2010, Mylan notified us that it had filed an abbreviated new drug application with the FDA seeking approval to market a generic version
of Sutent and challenging on various grounds the Sutent basic patent, which expires in 2021, and two other patents that expire in 2020 and
2021, respectively. In June 2010, we filed suit against Mylan in the U.S. District Court for the District of Delaware asserting the infringement of
those three patents. The patent expiring in 2020 was dismissed from the case prior to trial. In October 2014, the court held that the two patents
expiring in 2021 were valid and infringed. In October 2014, Mylan appealed the decision to the U.S. Court of Appeals for the Federal Circuit. In
January 2016, the U.S. Court of Appeals for the Federal Circuit affirmed the District Court’s decision upholding the validity and infringement of
the two patents expiring in 2021.
EpiPen
In July 2010, King Pharmaceuticals, Inc. (King), which we acquired in 2011 and is a wholly owned subsidiary, brought a patent-infringement
action against Sandoz, Inc., a division of Novartis AG (Sandoz), in the U.S. District Court for the District of New Jersey in connection with
Sandoz’s abbreviated new drug application filed with the FDA seeking approval to market an epinephrine injectable product. Sandoz is
challenging patents, which expire in 2025, covering the next-generation autoinjector for use with epinephrine that is sold under the EpiPen
brand name.
Toviaz (fesoterodine)
We have an exclusive, worldwide license to market Toviaz from UCB Pharma GmbH, which owns the patents relating to Toviaz.
Beginning in May 2013, several generic drug manufacturers notified us that they had filed abbreviated new drug applications with the FDA
seeking approval to market generic versions of Toviaz and asserting the invalidity, unenforceability and/or non-infringement of all of our patents
for Toviaz that are listed in the FDA’s list of Approved Drug Products with Therapeutic Equivalence Evaluations, commonly referred to as the
“Orange Book”. Beginning in June 2013, we filed actions against all of those generic drug manufacturers in the U.S. District Court for the
District of Delaware, asserting the infringement of five of the patents for Toviaz: three composition-of-matter patents and a method-of-use
patent that expire in 2019, and a patent covering salts of fesoterodine that expires in 2022. In June and July 2015, we settled with four of the
eight generic defendants. The trial relating to the remaining defendants occurred in July 2015, and we are waiting for a ruling from the court.
Tygacil (tigecycline)
In October 2013, we received notice of a Section 505(b)(2) new drug application filed by Fresenius Kabi USA LLC (Fresenius) for a tigecycline
injectable product. Fresenius asserts the invalidity and non-infringement of the basic patent for Tygacil that expires in April 2016, the
formulation patent for Tygacil that expires in 2029 and the polymorph patent for Tygacil that expires in 2030. In November 2013, we filed suit
against Fresenius in the U.S. District Court for the District of Delaware asserting the validity and infringement of the patents in suit. In
November 2015, we settled our claims against Fresenius on terms that permit Fresenius to launch a tigecycline injectable product in the U.S.
prior to the expiration of certain of the patents that were the subject of the challenge.
In November 2014, Mylan Laboratories Limited (formerly Agila Specialties Private Limited) (Mylan Laboratories) notified us that it had filed an
abbreviated new drug application with the FDA seeking approval to market a generic version of Tygacil. Mylan Laboratories asserts the
invalidity and non-infringement of the polymorph patent for Tygacil and the formulation patent for Tygacil. Mylan Laboratories has not
challenged the basic patent. In January 2015, we filed suit against Mylan Laboratories in the U.S. District Court for the District of Delaware,
asserting the validity and infringement of the polymorph patent and the formulation patent for Tygacil.
In addition, in September 2015 and December 2015, we received notices of Section 505(b)(2) new drug applications filed by each of Mylan
and Accord Healthcare Inc. (Accord) for tigecycline injectable products. Mylan and Accord assert the invalidity and non-infringement of the
polymorph patent for Tygacil, and two formulation patents for Tygacil that expire in 2028 and 2029, respectively. In October 2015, we filed suit
against Mylan in the U.S. District Court for the District of Delaware and in the U.S. District Court for the District of West Virginia asserting the
validity and infringement of the patents in suit. In February 2016, we filed suit against Accord in the U.S. District Court for the District of
Delaware and in the U.S. District Court for the Middle District of North Carolina asserting the validity and infringement of the patents in suit.
Precedex Premix
In June 2014, Ben Venue Laboratories, Inc. (Ben Venue) notified our subsidiary, Hospira, that it had filed an abbreviated new drug application
with the FDA seeking approval to market a generic version of Hospira’s premix version of Precedex and containing allegations that a patent
relating to the use of Precedex in an intensive care unit setting, which expires in March 2019, was invalid or not infringed. In August 2014,
Hospira and Orion Corporation (co-owner of the patent in suit) filed suit against Ben Venue, Hikma Pharmaceuticals PLC (Hikma), and West-
Ward Pharmaceutical Corp. in the U.S. District Court for the District of Delaware asserting the validity and infringement of the patent in suit. In
October 2014, Eurohealth International Sarl was substituted for Ben Venue and Hikma.
In June 2015, Amneal Pharmaceuticals LLC (Amneal) notified Hospira that it had filed an abbreviated new drug application with the FDA
seeking approval to market a generic version of Hospira’s premix version of Precedex and containing allegations that four patents relating to
the Precedex premix formulations and their use, all of which expire in 2032, were invalid or not infringed. In August 2015, Hospira filed suit
against Amneal in the U.S. District Court for the District of Delaware asserting the validity and infringement of the patents in suit.
In December 2015, Fresenius notified Hospira that it had filed an abbreviated new drug application with the FDA seeking approval to market a
generic version of Hospira’s premix version of Precedex and containing allegations that four patents relating to the Precedex premix
formulations and their use, all of which expire in 2032, were invalid or not infringed. In January 2016, Hospira filed suit against Fresenius in the
U.S. District Court for the Northern District of Illinois asserting the validity and infringement of the patents in suit.