Pfizer 2010 Annual Report Download - page 32
Download and view the complete annual report
Please find page 32 of the 2010 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
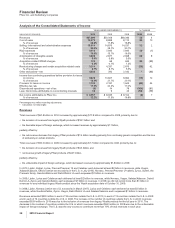
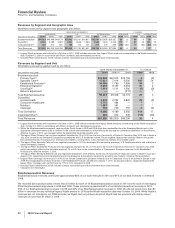
Financial Review
Pfizer Inc. and Subsidiary Companies
prevention indications after we submit our response to the “approvable” letters. In April 2009, Wyeth received approval in the EU for
CONBRIZA (the EU trade name for Viviant) for the treatment of post-menopausal osteoporosis in women at increased risk of
fracture. Viviant was also approved in Japan in July 2010 for the treatment of post-menopausal osteoporosis.
In July 2007, Wyeth received an “approvable” letter from the FDA with respect to its NDA for the use of Pristiq in the treatment of
moderate-to-severe vasomotor symptoms (VMS) associated with menopause. The FDA requested an additional one-year study of
the safety of Pristiq for this indication. This study was recently completed, and the results were provided to the FDA in December
2010.
In December 2005, we received an “approvable” letter from the FDA for our Vfend pediatric filing that set forth the additional
requirements for approval. In April 2010, based on data from a new pharmacokinetics study, we and the FDA agreed on a Vfend
dosing regimen for pediatric patients in three ongoing trials. We continue to work with the FDA to determine the next steps.
The Lyrica NDA for monotherapy treatment of GAD was withdrawn in December 2010.
In December 2010, in the interest of patient safety, we voluntarily withdrew Thelin for the treatment of PAH in markets where it is
approved. In addition, we discontinued clinical studies of Thelin worldwide for the treatment of PAH.
The NDAs for Fablyn (lasofoxifene) for the prevention and treatment of osteoporosis in post-menopausal women and for the
treatment of vulvar and vaginal atrophy have been withdrawn. We are exploring strategic options for Fablyn, including but not limited
to out-licensing or sale.
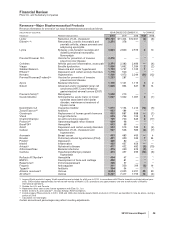
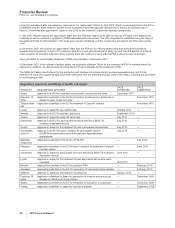
Regulatory approvals and filings in the EU and Japan:
PRODUCT DESCRIPTION OF EVENT
DATE
APPROVED
DATE
SUBMITTED
Sutent Approval in the EU for treatment of pancreatic neuroendocrine tumor December 2010
Prevenar 13
Adult
Application submitted in the EU for prevention of pneumococcal disease in
adults 50 years of age and older
December 2010
Taliglucerase
alfa
Application submitted in the EU for treatment of Gaucher disease November 2010
Lyrica Approval in Japan for neuropathic pain October 2010 —
Xalatan Approval in the EU for pediatric glaucoma September 2010
Torisel Approval in Japan for renal cell carcinoma July 2010 —
Genotropin Approval in the EU for adult growth hormone deficiency (Mark VII
multidose disposable device)
July 2010 —
Viviant Approval in Japan for the treatment of post-menopausal osteoporosis July 2010 —
atorvastatin
calcium
Approval in the EU for type II variation for atorvastatin calcium
(SORTIS and associated names) for pediatric hyperlipidemia/
dyslipidemia
July 2010 —
tafamidis
meglumine
Application submitted in the EU for ATTR-PN — July 2010
Macugen Application submitted in the EU for type II variation for treatment of diabetic
macular edema
— June 2010
Genotropin Approval in Japan for adult growth hormone deficiency (Mark VII multidose
disposable device)
June 2010 —
Lyrica Approval in Japan for the treatment of pain associated with post-herpetic
neuralgia April 2010 —
Revatio Application submitted in the EU for pediatric PAH — February 2010
Apixaban Application submitted in the EU for prevention of venous thromboembolism — February 2010
Xalacom Approval in Japan for the treatment of glaucoma January 2010 —
Prevenar 13
Infant
Application submitted in Japan for prevention of invasive pneumococcal
disease in infants and young children
— December 2009
Xiapex Application submitted in the EU for treatment of Dupuytren’s contracture — December 2009
Toviaz Application submitted in Japan for overactive bladder — September 2009
30 2010 Financial Report