Pfizer 2005 Annual Report Download - page 49
Download and view the complete annual report
Please find page 49 of the 2005 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
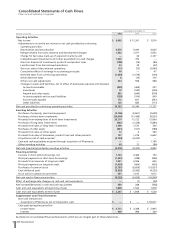
48 2005 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
Act). The Jobs Act created a temporary incentive for U.S.
corporations to repatriate accumulated income earned abroad by
providing an 85% dividend-received deduction for certain
dividends from controlled foreign corporations, subject to various
limitations and restrictions including qualified U.S. reinvestment
of such earnings. In addition, in 2005, we recorded a tax benefit
of $586 million related to the resolution of certain tax positions
(see Note 7D, Taxes on Income:Tax Contingencies).
Amounts are reflected in the preceding tables based on the location
of the taxing authorities. As of December 31, 2005, we have not
made a U.S. tax provision on approximately $27 billion of
unremitted earnings of our international subsidiaries. As of
December 31, 2005, these earnings are intended to be permanently
reinvested overseas. Because of complexity, it is not practical to
compute the estimated deferred tax liability on these permanently
reinvested earnings. On January 23, 2006, the IRS issued final
regulations on Statutory Mergers and Consolidations, which impact
certain prior period transactions. The regulations could result in
benefits ranging from approximately $75 million to $214 million
in the first quarter of 2006 subject to certain management decisions.
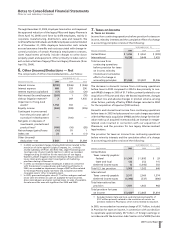
B. Tax Rate Reconciliation
Reconciliation of the U.S. statutory income tax rate to our effective
tax rate for continuing operations before the cumulative effect
of a change in accounting principles follows:
YEAR ENDED DEC. 31,
__________________________________________________
2005 2004 2003(a)
U.S. statutory income tax rate 35.0% 35.0% 35.0%
Earnings taxed at other than
U.S. statutory rate (19.5) (18.3) (53.2)
U.S. research tax credit (0.7) (0.6) (3.1)
Repatriation of foreign
earnings 14.4 ——
Resolution of certain tax
positions (5.1) ——
Acquired IPR&D 5.0 2.7 54.2
Litigation settlement
provisions ——13.7
All other—net 0.6 0.2 3.1
Effective tax rate for income
from continuing operations
before cumulative effect of
a change in accounting
principles 29.7% 19.0% 49.7%
(a) The large component percentages in 2003 reflect lower income
from continuing operations in 2003 due to the impact of the
Pharmacia acquisition.
We operate manufacturing subsidiaries in Puerto Rico and Ireland.
We benefit from Puerto Rican incentive grants that expire
between 2013 and 2023. Under the grants, we are partially
exempt from income, property and municipal taxes. Under Section
936 of the U.S. Internal Revenue Code, Pfizer is a “grandfathered”
entity and is entitled to the benefits under such statute until
September 30, 2006. In Ireland, we benefit from an incentive tax
rate effective through 2010 on income from manufacturing
operations.
The U.S. research tax credit is effective through December 31, 2005.
For tax years beginning after December 31, 2005, the research
credit has been suspended. For a discussion about the repatriation
of foreign earnings, see Note 7A, Taxes on Income:Taxes on
Income and for a discussion about the resolution of certain tax
positions, see Note 7D, Taxes on Income: Tax Contingencies.The
charges for acquired IPR&D in 2005, 2004 and 2003 are not
deductible. In addition, the litigation settlement provisions of $1.4
billion recorded in 2003 either are not deductible or are deductible
at rates lower than the U.S. statutory rate.
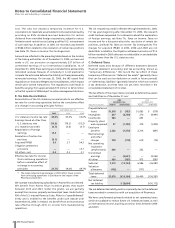
C. Deferred Taxes
Deferred taxes arise because of different treatment between
financial statement accounting and tax accounting, known as
“temporary differences.” We record the tax effect of these
temporary differences as “deferred tax assets” (generally items
that can be used as a tax deduction or credit in future periods)
or “deferred tax liabilities” (generally items for which we received
a tax deduction, but that have not yet been recorded in the
consolidated statement of income).
The tax effects of the major items recorded as deferred tax assets
and liabilities as of December 31 are:
2005 2004
DEFERRED TAX DEFERRED TAX
_____________________________ _____________________________
(MILLIONS OF DOLLARS) ASSETS (LIABILITIES) ASSETS (LIABILITIES)
Prepaid/deferred
items $1,318 $ (753) $1,085 $ (579)
Intangibles 857 (8,748) 270 (9,991)
Inventories 583 — 693 —
Property, plant
and equipment 87 (1,183) 279 (1,402)
Employee
benefits 2,282 (1,376) 2,314 (891)
Restructurings
and other
charges 729 (118) 619 (74)
Net operating
loss/credit
carryforwards 406 — 353 —
Unremitted
earnings —(2,651) —(3,063)
All other 950 (335) 973 (581)
Subtotal 7,212 (15,164) 6,586 (16,581)
Valuation
allowance (142) — (177) —
Total deferred
taxes $7,070 $(15,164) $6,409 $(16,581)
Net deferred
tax liability $(8,094) $(10,172)
The net deferred tax liability position is primarily due to the deferred
taxes recorded in connection with our acquisition of Pharmacia.
We have carryforwards primarily related to net operating losses
which are available to reduce future U.S. federal and state, as well
as international income, expiring at various times between 2006
and 2025.