Pfizer 2005 Annual Report Download - page 45
Download and view the complete annual report
Please find page 45 of the 2005 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
44 2005 Financial Report
Notes to Consolidated Financial Statements
Pfizer Inc and Subsidiary Companies
which has been allocated to our Human Health segment. Neither
of these items is deductible for tax purposes. In February 2006,
Eraxis was approved by the FDA.
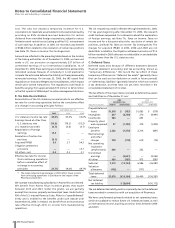
On April 12, 2005, we completed the acquisition of all outstanding
shares of Idun Pharmaceuticals, Inc. (Idun), a biopharmaceutical
company focused on the discovery and development of therapies
to control apoptosis, and on August 15, 2005, we completed the
acquisition of all outstanding shares of Bioren Inc. (Bioren), which
focuses on technology for optimizing antibodies. The aggregate
cost of these and other smaller acquisitions was approximately
$340 million in cash (including transaction costs) for 2005. In
connection with these transactions, we expensed $262 million of
IPR&D, which was included in Merger-related in-process research
and development charges.
On September 30, 2004, we completed the acquisition of
Campto/Camptosar (irinotecan), from sanofi-aventis for $525
million in cash (including transaction costs). Additional payments
of up to $63 million will be payable upon obtaining regulatory
approvals for additional indications in certain European countries.
In connection with the acquisition, we recorded an intangible asset
for developed technology rights of $445 million.
On February 10, 2004, we completed the acquisition of all the
outstanding shares of Esperion Therapeutics, Inc. (Esperion), a
biopharmaceutical company, for $1.3 billion in cash (including
transaction costs). The allocation of the purchase price includes
IPR&D of approximately $920 million, which was expensed in
Merger-related in-process research and development charges,
and goodwill of $239 million, which was allocated to our Human
Health segment. Neither of these items was deductible for tax
purposes.
In 2004, we also completed several other acquisitions. The total
purchase price associated with these transactions was
approximately $430 million. In connection with these transactions,
we expensed $151 million of IPR&D, which was included in
Merger-related in-process research and development charges,
and recorded $206 million in intangible assets, primarily brands
(indefinite-lived) and developed technology rights.
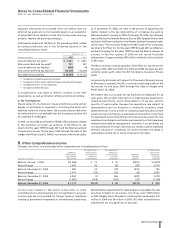
3. Dispositions
We evaluate our businesses and product lines periodically for
strategic fit within our operations. As a result of our evaluation,
we decided to sell a number of businesses and product lines and
we recorded certain of these results in Discontinued operations
for 2005, 2004 and 2003. All of the sales were completed as of
December 31, 2005.
•In the third quarter of 2005, we sold the last of three European
generic pharmaceutical businesses which we had included in our
Human Health segment and had become a part of Pfizer in April
2003 in connection with our acquisition of Pharmacia, for 4.7
million euro (approximately $5.6 million) and recorded a loss
of $3 million ($2 million, net of tax) in Gains on sales of
discontinued operations—net of tax in the consolidated
statement of income for 2005.
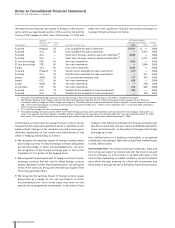
•In the first quarter of 2005, we sold the second of three
European generic pharmaceutical businesses which we had
included in our Human Health segment and had become a part
of Pfizer in April 2003 in connection with our acquisition of
Pharmacia, for 70 million euro (approximately $93 million)
and recorded a gain of $57 million ($36 million, net of tax) in
Gains on sales of discontinued operations—net of tax in the
consolidated statement of income for 2005. In addition, we
recorded an impairment charge of $9 million ($6 million, net
of tax) related to the third European generic business in
(Loss)/income from discontinued operations—net of tax in the
consolidated statement of income for 2005.
•In the fourth quarter of 2004, we sold the first of three
European generic pharmaceutical businesses which we had
included in our Human Health segment and had become a part
of Pfizer in April 2003 in connection with our acquisition of
Pharmacia, for 53 million euro (approximately $65 million). In
addition, we recorded an impairment charge of $61 million ($37
million, net of tax), relating to a European generic business
which was later sold in 2005, and is included in (Loss)/income
from discontinued operations—net of tax in the consolidated
statement of income for 2004.
•In the third quarter of 2004, we sold certain non-core consumer
product lines marketed in Europe by our Consumer Healthcare
segment for 135 million euro (approximately $163 million) in
cash. We recorded a gain of $58 million ($41 million, net of tax)
in Gains on sales of discontinued operations—net of tax in
the consolidated statement of income for 2004. The majority
of these products were small brands sold in single markets
only and included certain products that became a part of Pfizer
in April 2003 in connection with our acquisition of Pharmacia.
•In the second quarter of 2004, we sold our surgical ophthalmic
business for $450 million in cash. The surgical ophthalmic
business was included in our Human Health segment and
became a part of Pfizer in April 2003 in connection with our
acquisition of Pharmacia. The results of this business were
included in (Loss)/income from discontinued operations—net
of tax.
•In the second quarter of 2004, we sold our in-vitro allergy and
autoimmune diagnostics testing (Diagnostics) business, formerly
included in the “Corporate/Other” category of our segment
information, for $575 million in cash. The Diagnostics business
was acquired in April 2003 in connection with our acquisition
of Pharmacia. The results of this business were included in
(Loss)/income from discontinued operations—net of tax.
•In the second quarter of 2003, we completed the sale of the
hormone replacement therapy femhrt, formerly part of our
Human Health segment, for $160 million in cash with a right
to receive up to $63.8 million contingent on femhrt retaining
market exclusivity until the expiration of its patent. We recorded
a gain on the sale of this product of $139 million ($83 million,
net of tax) in Gains on sales of discontinued operations—net
of tax in the consolidated statement of income for 2003.
•In the first quarter of 2003, we sold the oral contraceptives
Estrostep and Loestrin, formerly part of our Human Health
segment, for $197 million in cash with a right to receive up to
$47.3 million contingent on Estrostep retaining market
exclusivity until the expiration of its patent. We recorded a gain
on the sale of these two products of $193 million ($116 million,